
Are the LCHF experts correct about biochemistry and cancer with respect to food dogma?
NOPE
NON PATHOLOGIC WARBURG SHIFT = cancer cells tend to convert glucose to lactate despite the presence of oxygen. Warburg called this phenomenon ‘aerobic glycolysis’, a term that is now synonymous with the ‘Warburg effect.’
The ‘Pasteur effect’, is where oxygen inhibits glycolysis, or conversely, hypoxia or a pseudohypoxia stimulates glycolysis. This last effect is why diabetes and higher Hba1C’s are seen with nnEMF of all types. Why? All nnEMF stimulate and cause pseudohypoxia in all cells lines but the effect is different in cell lines. High HbAIc’s are very dangerous to tissues with high metabolic rates that use the TCA cycle for beta-oxidation.
So are there any cells that are obligatory anaerobic glycolytic cells that are not cancerous in healthy people? Yes, there are and they all have one thing in common. Theses tissues exist within a life with low oxygen demand and therefore they do not make a lot of ROS/RNS free radicals to operate. It seems they do not need many electrons from ECT transport but they seem to crave protons. On the surface this seems like an unusual set of circumstances but it is not. Some of these cells do not have mitochondria and the ones that do hardly ever use the TCA or urea cycle. Obligatory glycolytic cells in normal tissues exist with autophagy and apoptotic intact as growth controls.
When we lose control of autophagy and apoptosis cells become at risk for having to use a PATHOLOGIC Warburg metabolism that has been the calling card for oncogenesis.
All somatic cells in humans are committed to self-sacrifice to protect the germ line. This is why apoptosis evolved initially. The key is not all cells share the same risk in this game of life. Some cells lack mitochondria or good blood flow, and as a result, they need glucose to run basic energy programs to remain biotic. This shows you that life is not wholly dependent on a lot of energy flux as we all believe. Is there something else that cells who have growth control who need glucose can live off of? There is. Remember these obligatory glycolytic cells don’t stop working during starvation either because they use gluconeogenesis for biosynthesis. What feeds them the information to know how to operate in this way? The information from solar light via the electron and proton spin, is the answer. These are the cells in the body that use information quanta more than they need energy quanta to survive.
What cells are these? Red blood cells, renal medullary cells, and certain cells in the retina called Muller cells and the cones used for color vision.
It gets more interesting. Warburg himself, and his team, also noted that normal mammalian retinal explants displayed obligate aerobic glycolysis normally. In other words, the retina, under normal solar conditions exhibits a metabolism that is similar to cancer cells. This was shocking to me when I first heard it 25 years ago. Why is that?
Why would our eyes and cancer cells share a metabolism?
This retinal finding, however, did not fit neatly with Warburg’s beliefs about cancer pathogenesis. In fact in the 1920’s Warburg’s critics used this against him and his thesis at every turn. Most of the critics and skeptics convinced him and his team that it HAD TO be attributed to an experimental artifact. Was it really caused by this, or was it related to the fact that all these obligate NORMAL glycolytic cells have to work this way for a reason? Was the reason due to the retina being heavily involved in light transduction metabolism? Might it be any cell that is using obligate glycolysis does so as a safety fuse because they are doing something unusual with incident light waves? Several decades thereafter, researchers with better tools confirmed that the mammalian retina indeed displays a strong NON-pathologic Warburg effect. the non pathologic Warburg shift does not allow for serious growth or proliferation. It can keep growth and metabolism under control. This is why you should never pay attention to critics/skeptics. Warburg’s observations were correct. He was never able to explain them and today I am going too in this blog using quantum biology of mitochondrial function.

REALLY?
HOW?
Might we be able to use this unusual finding to learn more about the biophysics and the quantum biology of human beings? I think the answer is unquestionably yes. Cancer researchers today routinely report that the Warburg metabolism is unique to cancer. Nothing could be further from the truth when you examine the data of the human retina. Today we know the Warburg effect is widely described in other cell types, namely embryonic stem cells, human T lymphocytes, neutrophils, dendritic cells and macrophages. This should begin to peak your interest after my April 2018 webinar. Are all these cell types deuterium bombs? Ye,s they are for the most part.
All life on Earth is believed to use adenosine triphosphate (ATP) to transfer energy. After the April 2018 webinar you now know definitively, I do not hold this view point. Let me give you a quick review. ATP is generated via two related metabolic pathways: OX-PHOS and glycolysis. Glycolysis converts a single molecule of glucose into two molecules of pyruvate, generating two ATP molecules. The final step requires pyruvate kinase (PK), which exists as several isoforms, notably PKM1 and PKM2 in the cytosol. In the presence of oxygen, pyruvate is usually converted to acetyl CoA, which then enters the Krebs cycle in the matrix, forming electron donors for OX-PHOS, generating approximately 32 net ATP molecules. When oxygen is scarce (pseudohypoxia) or falls short of demand, ECT is slowed. When ECT is slowed or absent, autophagy becomes impossible or unlikely, and pyruvate is shunted away from OX-PHOS in the TCA cycle and is converted into lactate by lactate dehydrogenase (LDH) in the cytosol to regenerate nicotinamide adenine dinucleotide (NAD+). Remember NAD+ wants all its H in the H+ form from the matrix. It does this because it needs the information of the light photon to transfer to the orbital angular momentum of the proton. This is the electron carrier of cytochrome one that deliever electrons to oxygen. Each electron carries photon energy and information as you learned in April 2018 webinar.
Each of the steps within the glycolytic pathway is catalyzed by a specific enzyme or enzyme complex. This is not important right now. Some of these enzymes have a role in protein transcription regulation, cell motility and apoptosis regulation. Cytochrome c is the controlling mechanism for human apoptosis. If apoptosis is operational it is impossible to get cancer. Apoptosis is made efficient by IR and UVa light. This is important and critical for the mitochondriac to know and understand how it fits into the story of quantum biology using quantum thermodynamics. Information to these enzymes is far more important than energy flux. It seems the process of apoptosis needs more information to operate than energy flux.
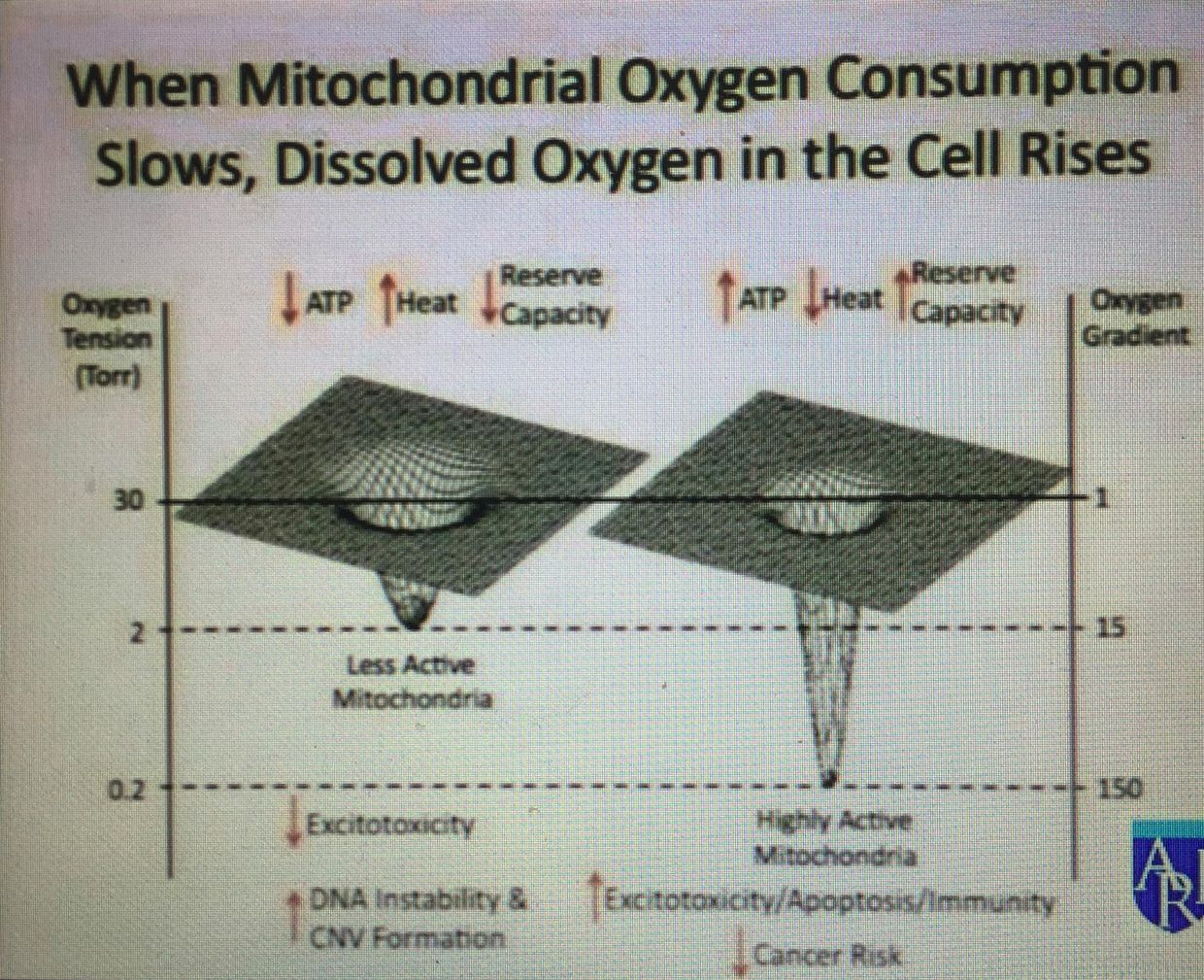
I wrote the EMF 4 blog post long ago to teach you about the pentose phosphate pathway (PPP). I did this for many reasons. I was trying to get you to realize that a cell has many trap doors, or quantum demons, it can use when the environment changes light and temperature and calls for them to be used. Anytime light and temperture what also changes in the environment? Oxygen tensions do. Mitochondria pay DEEP attention to that change as a picture below shows. These are the metabolic doors that appear to vanish when they are not needed. When they are needed they can be massively amplified, as you saw in the May 2018 webinar. When a cell is missing IRA and UVA light is can cause a massive amplification of things that a cell should rarely see and this is why cancer is often the result of a loss of control of information quanta. The reason for the observed biochemical changes are always biophysical and way below a researchers ability to find them because they have no idea they are there, much less what they do in cells. You cannot find or control for things you do not even know that matter.
In proliferating cells, glucose not only produces ATP, but also provides metabolic intermediates for biosynthesis. Intracellular glucose can also be directed towards biosynthesis: into the pentose phosphate pathway to generate nucleotides and nicotinamide adenine dinucleotide phosphate (NADP), or gluciose can be reduced, to make the amino acids, serine and glycine. This was the serine glycine interconversion pathway I mentioned in the Reality series. Serine and glycine can link and jump from glycolysis at the phosphoglycerate step. The enzyme phosphoserine phosphatase is the final step in glucose-serine conversion. The PPP has to use NADPH. The H has to be H+ because it is the key information quibit. It carries more information from the environment to the matrix of a cell than dueterium can. This is exactly what I said above about NADH. This detail is critical to understand.
The ability to oscillate between biosynthesis, energy production, and information transfer is quite unique in cell biology. It provides proliferating tissues with a powerful metabolic strategy known as the ‘metabolic budget system.’ This is kind of like a carburator controlling the flow of gas into an engine cyclinder where a spark plug is ready to produce power. This phenomenon actually goes hand in hand with what Warburg described in his papers on these cell effects. This strategy can be viewed as the presence of the Warburg effect in a tissue using glucose for biosynthesis. Such a phenomenon, is rarely noted in a non-proliferating tissue, such as the retina or RBC. These two tissues were my focus in the last two years in the my Vermont talks.
The glycine serine pathways (talked about in survivor soup blog) are needed in making all the opsins proteins in the body. Few people seem to know this fact either. Pyruvate kinase isoform M2 (PKM2) and hypoxia- inducible factor-1 (HIF-1) are the key regulators of the non pathologic and the pathologic Warburg effects. HIF-1 is a protein that stabilizes 3D protein conformation. The control of oxygen is under the control of local tissue metobolism. Methionine cycling is critical in the angiogenesis signal in tissues. As the TCA and urea cycle slow, methionine is raised as a collateral effect. This is incredibly important in the opsin systems because of the potential of photooxidation from the highly powered light like the blue light at night. HIF is induced in hypoxic or pseudohypoxic environments.
Pyruvate kinase (PK) is a glycolytic enzyme that catalyses the conversion of phosphoenolpyruvate into pyruvate, generating one molecule of ATP in the rate-limiting final step of glycolysis. There are four isoforms of PK in mammals: L – liver, R – red blood cell, M1 – adult (muscle and brain) and M2 – embryonic and tumor. Cells needs multiple isoforms to control the metabolic rate of each tissue differently to modulate growth. How do they do this? Each one allows a different level of deuterium to get into the Kreb’s bicycle area of each tissue.
GEEK PART: Here is where it gets interesting and complex for the non scientist. Uniquely, PKM2 has an allosteric pocket not present in the other isoforms that permits binding to phosphotyrosine peptides and fructose 1,6 biphosphate. This structural configuration renders PKM2 vulnerable to various regulatory inputs. Whereas PKM1 forms a stable, constitutively active tetramer, PKM2 oscillates between the active tetrameric and the inactive dimeric (or monomeric) forms. The dimeric form has a low affinity for the substrate phosphoenolpyruvate and lower activity than the tetrameric form. When the dimeric form dominates, phosphoenolpyruvate conversion becomes inefficient, and as a consequence glycolytic intermediates upstream of phosphoenolpyruvate accumulate and are available for biomass synthesis and cell proliferation. This is why cancer has to depend on glycolytic intermediates………If the TCA/urea cycle is non operational this pathways to biosynthesis and survival is its only choice unless the cell can recpature control of autophagy or apoptosis in some way quickly.
As the glycolytic intermediate, fructose 1,6 biphosphate accumulates, the reaction favors conversion of the dimeric form back to the tetrameric form and pyruvate is produced efficiently again. These regulations of PKM2, labelled as the ‘metabolic budget system’, has been proposed to control the anabolic biosynthesis versus energy production in tumor metabolism.
Guess what I believe controls this process? The information in protons and electrons that have had photonic information transferred from the quantum spin number to the orbital angular momentum (OAM). Oxygen tension also play a role as the picture below shows. This is how intelligence is built in atoms in these cells. Apoptosis especially uses this mechanism, as I laid out in the May 2018 webinar.
High PK activity leads to depletion of glycolytic intermediates available for biosynthesis and therefore impairs cellular proliferation and growth in obligate glycolytic cells like the RBC’s in the blood. This is why so many cancers are associated with anemia. Ironically, the tumor tries to get around the anemia by increasing angiogenesis factors released from the surface of arteries by rising levels of anion amino acids with sulfur. As anemia worsens the chance of apoptosis working well drop signficantly because RBC’s are what carry IRA and UVA light to restore apoptosis efficiency to the mitochondria in our tissues.
The situation I am laying out here would not be a good state for a cancer cell. There is a lesson here in cancer prevention. Cancers by default, need a ton of glycolytic intermedates to make things it needs to divide, so it always wants high activity of PK. To keep that chronic growth activity in cancer, autophagy has to be impaired and apoptosis has to be eliminated completely while ECT flow has to be brisk. How does cancer accomplish this? You cells have to bring more oxygen to the cancer cell and the excess oxygen pulls electrons briskly to oxygen because of its electronegativity. This augments ECT even in a broken mitochondria. What causes this amplification of oxygen/angiogenesis when a cell is using glycolysis and the PPP? Methionine cycle kinetics do. Methionine is an essential amino acids loaded with sulfur. It turns out when the TCA and urea cycle kinetics are broken, methionine levels rise and becomes a relative toxin because as it rises in a tissue it cause angiogenesis. Cells forced to use the two older metabolic pathways should never have high oxygen tensions because this always leads to a proliferative pro-growth signal from the mitochondria to the nuclear genome.
It also explains why glucose is upregulated in cancer cells or in obligate glycolytic cells. It is not because cancer cells only use glucose and glutamine, it is because they cannot use the TCA and urea cycle because their kinetics are blocked for some reason. Those two cycles need both autophagy or apoptosis to have some minimal efficency to protect from uncontrolled growth and control of methionine. The information of how to use these metabolic pathways in this way is buried in the type of protons used in the pathways. Hydrogen isoforms is the optical switch in these utilization of any metabolic pathway in a cell. When the signal is disordered cells begin to use older evolutionary pathways when oxygen tension is too high. When this occurs, deuterium leaches from cell membranes into the matrix where Kreb’s bicycle sits, and it destroys TCA and urea cycle kinetics faster than anything on Earth. The amount of deuterium let in by a cell via uncoupling proteins and oxygen levels determines what metabolic path will be chosen in an environment: It also determines what pathway is optimal for a non proliferation or proliferation tissue.

In the RBC’s and retina evolution controlled the choice by making mitochondria and blood flow scarce. Given the situation, we certainly do not want light from the sun causing uncontrolled growth in our eye, because then vision is obscured. But this i sexactly what happens in a diabetic retina. To avoid this situation, the body uses the control of proton flow in the matrix and ATPase to control these pathways in a non proliferative way. Not all the cells in the retina use the TCA level because of this. Muller cells happen to be one of these cells (pic above).
This is why RBC’s and parts of the retina have no mitochondria and little blood flow in the fovea where sharp color vision occurs. It is shocking to see such a high energy demand tissue rely on older metabolic pathways to limit oxygen to control growth. Could this be why people with faulty mitochondria develop sleep apnea too? Is it a protective phenomena built by nature? I think so.
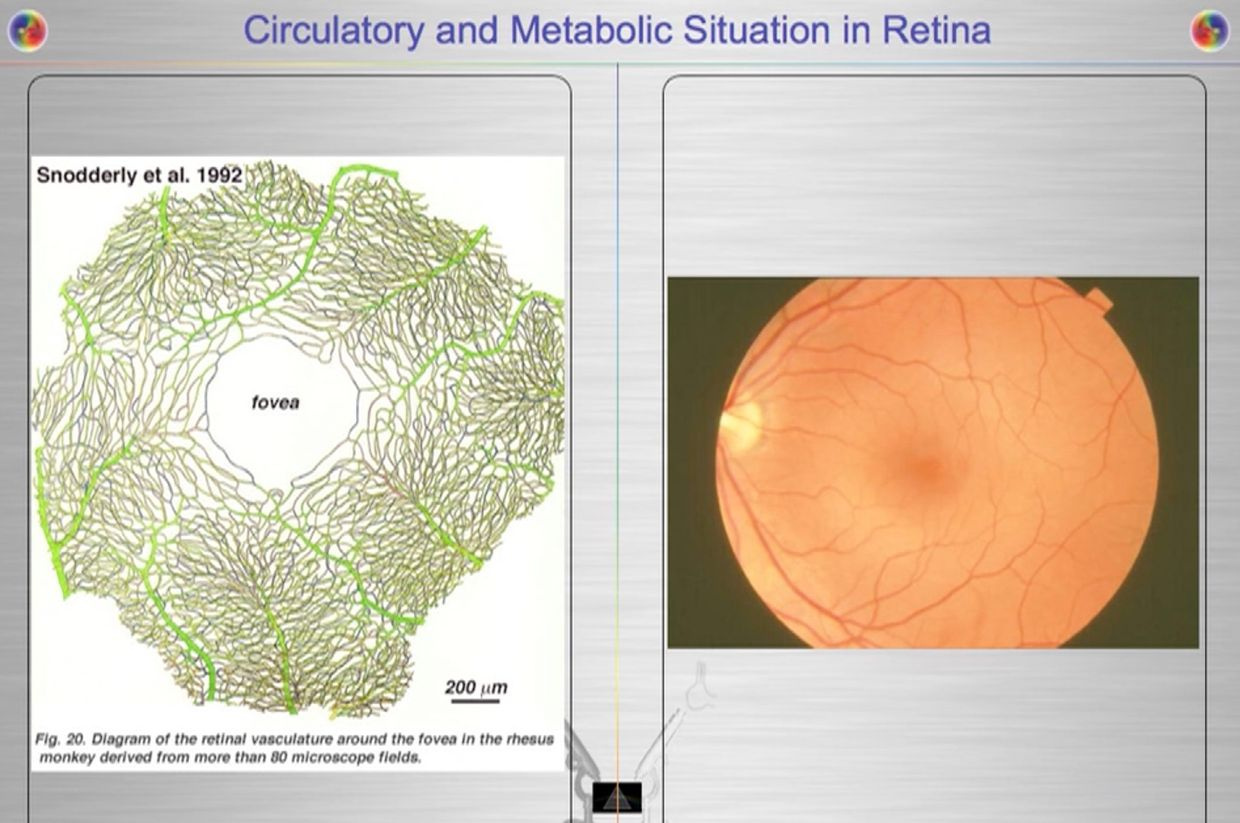
The retina has prodigious energy demands to maintain the neurons in an excitable state for phototransduction and neurotransmission, in addition to the maintenance of normal cellular function. Any cells involved in light transfer seems to have a high concentration of lactate in the venous blood while also having low oxygen tensions. This is a reason I use lactated Ringer’s solutions when I am doing mitohacks involving light frequecies when my BUN/creatine ratios are altered. These labs are redox proxy’s telling the mitohacker that there is hypoxia in a cell and we better be careful in how we construct our experiment. I had to leanr this lesson the hard way many times in hacks. IV LR solution is an awesome hack for people with chronic BUN/creatine decline. You can learn a lot about proton information capapbilities when you use an electroretinogram to educate your mitochondriac perspective (pic below).

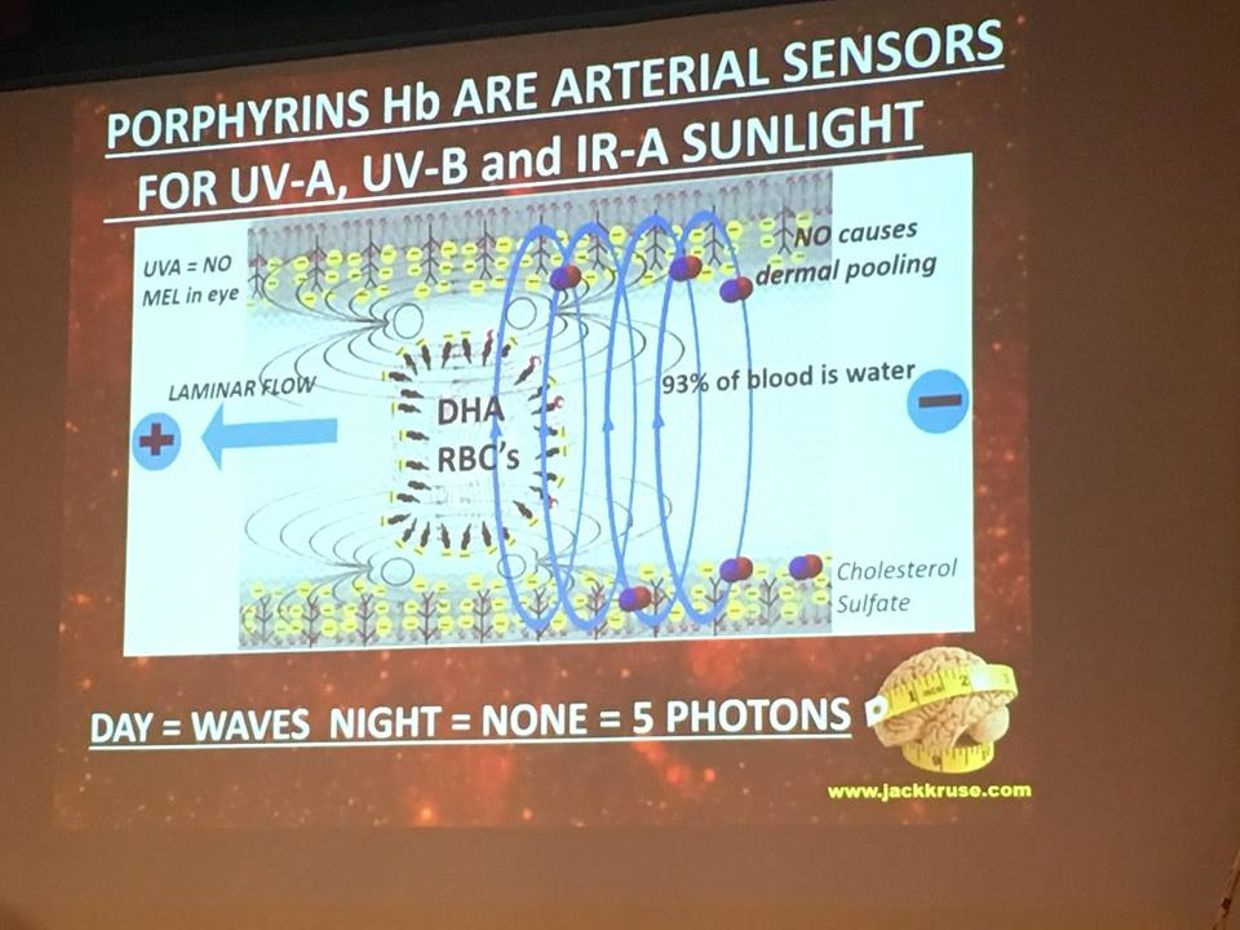
Your opthalmic artery feeds the retina a lot more than energy and light to the eye and brain. The retina is built backwards to slow light down to harvest a massive amount of its information to build things and to know things about the environment we inhabit. It can be seen on direct examination of the eye. Recall that 60% of your circulating blood volume passes through this artery in sunlight. This is a massive way to add energy and information to the system of the eye and brain. The blueprint of retinal physiology is telling me that the retina is more interested in information in light waves than its energy because the total amount of oxygen extracted from choroidal (RPE) and retinal blood flow combined has only accounted for partial accounting of the oxidation of glucose in hacks I have done in this tissue. I found out that the same experiments have been done in many mammal retina’s because of Warburg’s original papers were where the obligate glycolysis was first observed in science. In pigs complete oxidation of 37% of all the extracted glucose, were found in their retina and this reflects the high glycolytic activities in the pig retina when light was present. In pigs, the majority of the glycolytic substrate was derived from the choroidal circulation (RPE), indicating there was greater metabolic activity in the outer retina where vision DID not happen. This is where melanopsin was located. This was a big clue to me why blue light is devasting to growth and metabolism choices in an animal. It also explained why cancer was a serious consequence of blue light at night.
When I learned about these counterintuitive concepts I knew immediately the reason why it localized to the outer part of the retina. This is where melanopsin is plentiful. All opsins are connected to a Vitamin A moiety. Diurnal mammals and nocturanl mammals have this but the covalent binding of Vitamin A to the melanopsin is radically different. I began to realize why. That bond was stronger to offset the risks of light at night when you are nocturnal to control growth. This opsin needed Warburg protection to operate safely in the mammalian retina. It made perfect sense when I thought about the consequences if the systems pieces fell apart. Blue light is highly powered and will make a lot of ROS and not much ATP. Lowered ATP means lowered antioxidant spikes to cause swelling, which is the stimulus to growth. This mechanism had to have tight controls otherwise cancer is very likely in the animal.
Excessive ROS production is dangerous to the retina because it will stimulate apoptosis and thin the retina . So what did Mother Nature do to protect us?
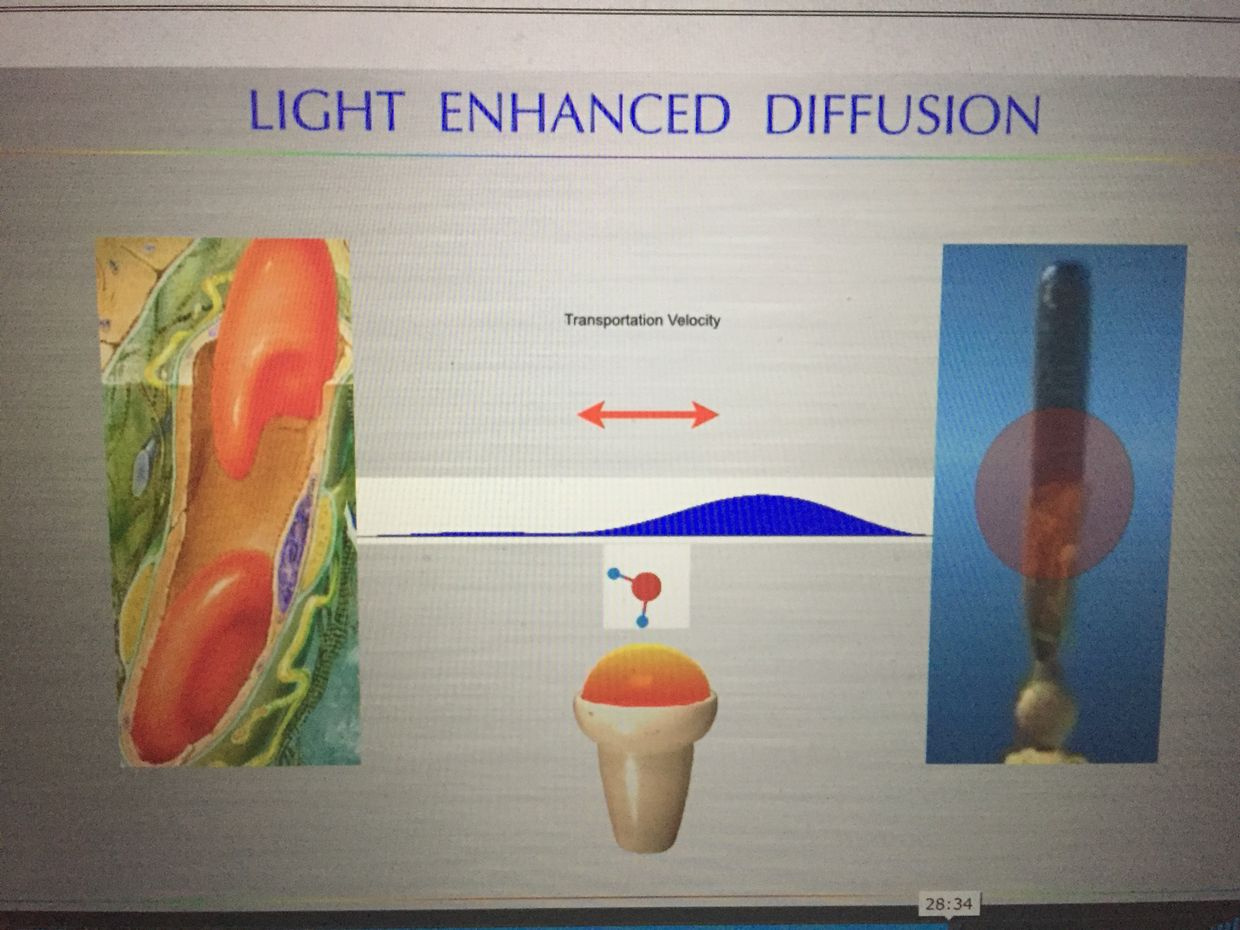
As I laid out in my Vermont 2017 talk, she put the photoreceptor far away from the blood supply. This put a ton of blood, water, and deuterium between the two positions. This allowed massive information transfer from sunlight to the H+ protons while the heavier atomic mass of deuterium moves waves of blood through tiny vessels more quickly using solitons. The ROS of the blue light was quenched by the presence of 42% red light and because the cells in this region of the retina had to be obligate glycolytic cells that would make low levels or ROS to protect from an apoptotic signal. Mother nature is nothing short of amazing. All of these quantum thermodynamic actions formed the basis of my Vermont 2017 talk.
You might want to go back and relisten to the Vermont 2017 video now that you have this understanding now.
VERMONT 2017 VIDEO
CITES:
https://onlinelibrary.wiley.com/doi/pdf/10.1111/ceo.12462
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2897203/ Paper on glycolsis flux and O2 with PPP