Did you know albumin is also a ‘dynamic’ fluorophore protein in the blood? Its ability to absorb UV light changes with temperature and pH. After Vermont 2018 talk this should stop you dead in your tracks. What might it mean? Research has shown albumin has 4.66 percent TYROSINE and 0.19 percent tryptophan at a pH of 2.
Lerner and Barnum noted in 1946 that a shift in the maximum absorption of serum albumin to longer wavelengths when the pH was changed from 2 to 10 in the blood plasma. As the temperature rises, molecular vibrations increase which results in the ability of water to ionize and form more hydrogen ions (H+). As a result, the pH will drop.
- The local environment (pH/temperature)of the aromatic amino acids can have an effect on their spectra. This means that tryptophan will have an emission peak at lower wavelengths when buried within the hydrophobic inner regions of a protein while tyrosine will often transfer its energy to adjacent tryptophan amino acids. Ionized tyrosinate, which forms when protons are lost from tyrosine as a result of increasing pH, will demonstrate wavelengths similar to tryptophan.
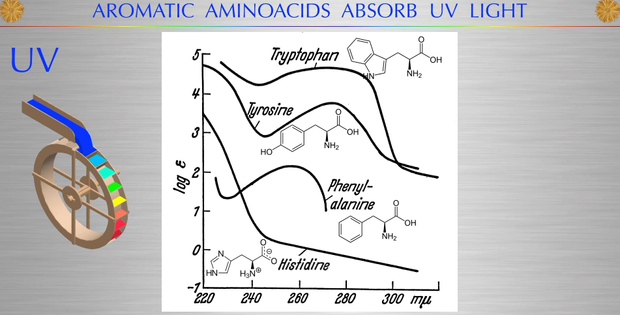
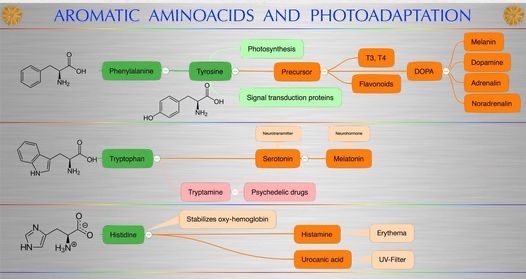
Phenylalanine and tyrosine are the simplest aromatic amino acids derived from alanine. Phenylalanine is an essential amino acid that our body cannot synthesize. This does not apply to tyrosine, which the body can synthesize only if there is a sufficient amount of phenylalanine. Tyrosine has one of the highest absorption spectra for UV light in biochemistry. Interesting huh?
The maximum and minimum absorption of tyrosine moves progressively to longer wavelengths in the ultraviolet range as the pH is increased above pH 7.0. This is why inflammation in the body lowers your ability to harvest UV light. It is also why people with high HS CRP often have low Vitamin D levels even when they live in a good solar environment and you have tan skin.
In solutions of pH 12.0, a second sharp absorption maximum appears at 240 nm, which is ascribed to the ionization of the phenolic group of tyrosine. The ultraviolet spectra of tryptophan shift only slightly with pH, while those of phenylalanine are relatively independent of pH. Tyrosine physiology is most sensitive to inflammation in the body.
pH decreases with an increase in temperature. You do know that UV light increases temperature the most on our surfaces huh? But this does not mean that water becomes more acidic at higher temperatures. A solution is considered acidic if there is an excess of hydrogen ions over hydroxide ions.
Does anyone want to guess what is made from tyrosine?
Thyroid hormones. Melanin, dopamine, adrenaline, and noradrenalin. Inflammation in your body lowers the production of all these chemicals.
Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of phenol functionality. It occurs in proteins that are part of signal transduction processes. It functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hydroxyl group can change the activity of the target protein or may form part of a signaling cascade via SH2 domain binding. This is a topologic change mediated via charge alterations that are very important. If you’ve carefully read the last few blogs this information should stir you.
DOES TYROSINE MATTER IN PLANTS TOO?
A tyrosine residue also plays an important role in photosynthesis. In chloroplasts (photosystem II), it acts as an electron donor in the reduction of oxidized chlorophyll. In this process, it loses the hydrogen atom (H+) of its phenolic OH-group. This radical is subsequently reduced in the photosystem II by the four core manganese clusters. Plants and the microbiome make tyrosine from the shikimate pathway and mammals usually get it from the conversion of phenylalanine from foods. That is another aromatic amino acid. When this conversion is made in humans it creates more water. That water is also special. When tyrosine is changed into all the catecholamines in our cells did you know it throws off water in the conversion? What might the purpose of this be to Nature?
The thyroid hormones triiodothyronine (T3) and thyroxine (T4) in the colloid of the thyroid also are derived from tyrosine in the anterior pituitary.
Tyrosine is also the precursor to the pigment MELANIN found in the RPE of your eye and the pigment of your skin.
Tyrosine (or its precursor phenylalanine) is needed to synthesize the benzoquinone structure which forms part of coenzyme Q10 that shuttles electrons from cytochrome 1 to cytochrome 3. This is part of the Q cycle I did an entire webinar on. Do you know that UVA and UVB products tend to inhibit ECT at this level too huh?
Tyrosine hydroxylase catalyzes the initial and rate-limiting step in the biosynthetic pathway of catecholamines including dopamine, noradrenaline, and adrenaline. Regulation of thyroid hormone activity is important for a variety of physiological and behavioral functions of these catecholamines.
The ultraviolet absorption spectrum of serum albumin was built to be dynamic to the solar spectrum for some reason. Not only that but it can be interpreted almost quantitatively on the basis solely of the tyrosine present in the protein.
Tyrosine makes some important things in our blood plasma, doesn’t it? Things that should make you go hum after my Vermont 2018 talk………..is this why dopamine can be made in the powerful sun and during CT when the sun is weak? The answer is Yep. It can be made in two ways so that mammals can dominate in all seasons.
Is this why studies have found tyrosine to be useful during conditions of stress, cold, fatigue, prolonged work, and sleep deprivation, with reductions in stress hormone levels? Yep. When you know better about the light you always do better than any food guru can teach you.
BATTERIES, CAPACITORS, AND CHARGES
Hydrogen bonding networks are very dynamic relative to the electromagnetic and charge environment they find themselves in. They are so dynamic that we cannot perceive how fast they adapt. It happens at femtoseconds. The fast adaptive behavior of these H+ networks in water, alone determines the dielectric constant of the medium they are within. Seasonal weather variations due to the sun drive these processes for biology because how light alters the charges of things inside of cells. Normal bulk water has a high dielectric value. Low pH aqueous environments (inflammation) contain more protons or fewer electrons and as a result, have a lowered dielectric value.
Alkaline pH also changes the dielectric constant in biological systems because of how charges evolve. The coherent domains or EZ water in cells has a dielectric constant of 160. The non coherent domains in water vary from 14 to 78. Charge conservation does not act like energy conservation ideas found in the laws of thermodynamics. Energy cannot be created or destroyed. Neither can charges.
Here is the key difference: Charge confirmation does not mean that individual positive and negative charges cannot be created or destroyed. Why? Electric charge is ONLY carried by subatomic particles such as electrons and protons, and those particles move in biological systems changing the dielectric potential of tissues as the charges move. Charging of water by sunlight creates a massive net negative charge in water’s coherent domains also called the exclusion zone (EZ). Charged particles that carry electrical & magnetic information can be created and destroyed in elementary particle reactions. CERN does this every day. So does the sun. Biology has yet to realize the implications of this in cells. People forget that light can change or alter the charge on biomolecules.
CHARGE IS A BIG DEAL IN BIOLOGY.
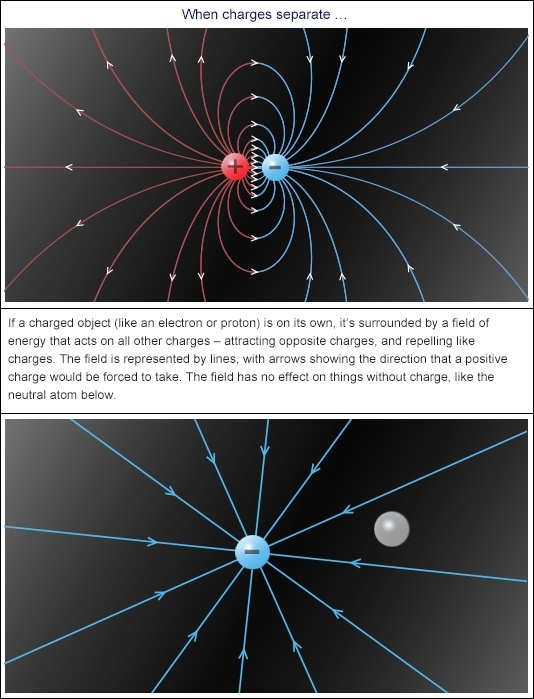
Trying to define charge is like trying to define love – you can only really describe the effect it has on other things. And both charge and love rest heavily on the “opposites attract” rule.
In the atoms and molecules that make up all matter, there are only two players when it comes to charge: protons with a positive charge, and electrons with a negative charge. Their charges are opposite, so they attract. And their charges are equal (both have a charge of 1), so when they get together they balance out each other’s charge, giving atoms an overall neutral (zero) charge.
There’s a yang to all that attraction yin — positive charges repel other positives, and likewise for negative charges.
That’s the accounting side of the charge story – now for the fun stuff: separating opposite charges to create electric fields.
The attraction between opposite charges means that they’re usually found stuck together, and pulling them apart always takes energy. Mitochondria separate electrons from protons in foods via electron chain transportation nanomachines.
Once they’re apart that energy gets stored in an electric field around each of the individual charges. The field instantly spreads out from the charge in all directions at the speed of light, and literally goes on forever.
But fields get weak with distance so the charge’s attractive/repulsive effect dies off pretty quickly. It’s the electric field of energy around them that lets isolated charges attract and repel each other without ever touching!
But batteries aren’t the only things that can keep charges separated, and make use of the electric field in between them. Capacitors are like a kind of temporary battery – they keep positive and negative charges separated by an insulator, with an electric field between and around them. Mitochondria should be thought of as a diurnal capacitor that tells time.
The more negative and positive charges you separate, the stronger the electric field that forms between and around them, and the more electrical energy that can be tapped. That separation of charge is what creates voltage, or potential difference.
Batteries get their voltage by having positive and negative charges separated – their positive and negative electrodes are separated by an insulating layer. Each time you use a battery, a bit more of the separated charge gets reunited via the circuit, and the electric field between the positive and negative electrodes gets a bit weaker. Rechargeable batteries pump the charges back to their separate sides during charging, strengthening the electric field all over again. Cells strengthen their electric fields when they are exposed to sunlight daily. The battery in cells are water based (EZ).
But batteries aren’t the only things that can keep charges separated, and make use of the electric field in between them. Capacitors are like a kind of temporary battery – they keep positive and negative charges separated by an insulator, with an electric field between and around them. Mitochondria should be thought of as a diurnal capacitor that tells time.
Touchscreens in phones and tablets act like capacitors when they use electric fields to detect your fingers. And it’s not just electronics that rely on capacitance – every living cell acts like a capacitor too.
Most electrical charge is found in water networks inside of cells. This is called the exclusion zone (EZ) in cells. This charge in the EZ can then be transferred to ions in cells to create signals in cells. Cells spend a lot of energy pumping charges (positive ions like potassium and sodium, and negative ions like chloride) through their cell membrane to make sure that inside the cell is more negative than outside. Neurons do it the other way around to generate action potentials.
That separation of charge means that there’s always an electric field, and therefore a voltage, across the cell’s plasma membrane. It’s called the transmembrane potential, and it controls the enzymes and gates in the membrane, so the cell can be in charge of what’s moving in and out. The state of water in sunlight sets the transmembrane potential. Most biology books miss this idea because they did not understand the work of Gilbert Ling.
So an individual electric charge will always have an electric field around it, spreading out forever. And if you move that charge, the electric field will move with it. But the entire universe— wide field doesn’t move instantaneously – the movement travels out through the field at the speed of light. Which isn’t surprising, because bounces in electric fields are exactly what makes light. Every kind of light – from radio and visible light right through to X— rays and gamma rays.
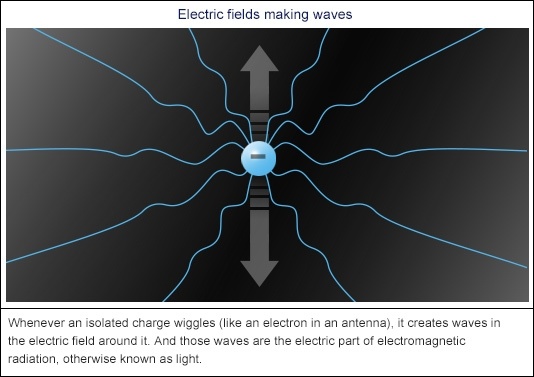
If a charge (say an electron) moves up, the electric field moves up too – at the speed of light. If the charge moves down again, the field moves back down at the speed of light. No matter what speed the charge is moving at, its electric field will follow it at the speed of light, so it takes a tiny fraction of time for the whole field to catch up. And if you make the charge move up and down (or side to side, or back to front) in a nice steady rhythm, the electric field around it forms waves – like the proverbial ripples on a pond – moving out in every direction at the speed of light. There’s one crest for every bounce of the electron.
And that’s exactly half the story of how moving charges make electromagnetic radiation.
The other half involves the ‘magnetic’ bit. Whenever an electric field moves, it automatically creates a magnetic field that mimics its moves but at a 90 degree angle. So if the electric field is bobbing up, it’ll create a magnetic field bobbing out to the side. When the electric field bounces down, a magnetic field will bounce out to the other side.
An oscillating electric and magnetic field caused by the jiggling of an electric charge are exactly what causes the radio and microwave signals that come out of antennas. Cells use a DC current to cause their oscillations. In tech gear the AC alters the charges and causes electrons to loosen in the metal antenna. When these electrons are dislocated, they are being forced to move side to side by the alternating current. AC current and DC current do not cause the same oscillatory pattern and this creates a problem for cells.
In fact every electric wire that’s hooked up to AC power on Earth will give off some radio waves at the frequency of the power source – that’s why electric appliances can interfere with TV & radio signals which is why airplane mode was invented. Cells where built around the DC power source of the sun because there is no RF or microwave radiation given off at the frequency of the power source.
A pure DC circuit doesn’t produce radio waves because it is impossible. A DC signal (or power) is unchanging, so there can be no light wave propagation going on. It is important to understand that fluctuating DC cannot exist – anything that fluctuates is actually AC. This is fundamentally why you hear me on podcasts say that Tesla AC power grid is the worse invention mankind has ever had and few people understand why I say it. Now you do. The NTP toxicity study of Nov 2018 is the cherry on top of this idea.
SUMMARY
Light alters charges. Light knocks electrons out of tyrosine. Tyrosine acts like an antenna in cells and helps facilitate charge motions in cells where it is found. Where does biological light come from to cause signaling?
Many studies have reported that biophotons arise from the many metabolic processes that occur within the cell (Grass et al., 2004; Tang and Dai, 2014; Salari et al., 2015; Mothersill et al., 2019; Van Wick et al., 2020; Zangari et al., 2021). The main source of biophotons is thought to be the mitochondria, where most of these metabolic reactions take place. All food was created by sunlight in photosynthestic webs. In mitochondria, the process is reversed and light captured in food stuffs can be liberated for signaling. In this way only, should food be thought of like a drug. From oxidative phosphylation of food in the mitochondria, then light is liberated from the solar created foods. For light to be created oxygen and ROS must be present. In particular, biophotons appear to result from the process of oxidative metabolism, the excitation and subsequent relaxation to a stable state of reactive oxygen species. The biophotons are likely to be absorbed by a number of chromophores within the cell, including tyrosine and its phenolic ring, porphyrin, flavinic, and pyridinic rings, lipid chromophores, aromatic amino acids (tyrosine) and cytochrome c oxidase. The communication can span to other cells via cytonemes.
Cytonemes are thin, cellular projections that are specialized for exchange of signaling proteins between cells.
This light absorption – either by the same or neighboring (also called bystander) cells – can then lead to a change in electrical activity (Mothersill et al., 2019; Zangari et al., 2021). Microtubules are also suspected to play a role in this process, being involved in the intracellular transmission of the signal (Tang and Dai, 2014; Mothersill et al., 2019). There is a distinct delay, from the time of biophoton production to absorption, called delayed luminescence; the length of this delay provides key information about the functional quantum status of the cell (Salari et al., 2015). The quantum status of the cell is a function of its redox potential. Redox potential is a function of the charged ability in the cell. It is intimately associated with the state of water production in mitochondria.
There are two striking features of biophotons in cells. First, they are emitted with a rather broad range of wavelengths, from ultraviolet to red and near infrared range (i.e., 200–950 nm). Such a broad range opens the possibility that particular wavelengths within that range are associated with different cellular reactions and different states of homeostasis (Dotta et al., 2014; Tang and Dai, 2014). Second, this self-generated light from neurons is not bright, not something that is detectable by the naked eye, nor even a relatively sensitive radiometer, but only with an ultra-sensitive light detection device, such as a photomultiplier or with a very specific histological stain (Zangari et al., 2021).
It has been estimated that the number of biophotons generated by a cell can vary anywhere between 2–200 photons/s/cm2(Tang and Dai, 2014; Salari et al., 2015). This level of emission appears to occur steadily, but the level is increased or decreased by an external stimulus for example, by man made light, or electrical stimulation, thermal or mechanical stress, the application of neurotransmitters like glutamate or the addition of an anesthetic or tetrodotoxin. It should be noted that both the biophoton intensity and wavelength can vary depending on the particular state of homeostasis (redox) of the cell. For example, there are distinct differences in both the numbers and wavelengths of biophotons evident between cancerous and non-cancerous cells (Tang and Dai, 2014; Salari et al., 2015).
Tyrosine absorbs light strongly in the 220nm-280nm range. This matches it to water absorption spectra of light as well. This should explain to you why solar light is imperative for diseases linked to chemicals made from tyrosine/phenylalanine.
Tyrosine contains a reactive hydroxyl group, thus making it much more likely to be involved in interactions with non protein atoms in cells. Tyrosine can be created from phenylalanine too. Tyrosine is an alpha-amino acid that is phenylalanine bearing a hydroxy substituent at position 4 on the phenyl ring. Phenylalanine is an essential amino acid in humans while tyrosine is non-essential. Besides its incorporation into proteins, the only function of phenylalanine in humans is its conversion to tyrosine. Tyrosine forms most of the stress neurotransmitters in the body. Light stress lowers the production of these chemicals in humans.
Exposure to excessive blue light, nnEMF, or higher heteroplasmy rates is associated with calcium efflux in mitochondria. As calcium effluxes rises it causes problems with neurotransmitter (NT) release; all NT’s need calcium ions in proper doses to release from synapses and work in neurons. In particular, tyrosine can be metabolized to produce hormones such as thyroxine and triiodothyronine or it can be metabolized to produce neurotransmitters such as L-DOPA, dopamine, adrenaline, or noradrenaline. Tyrosine can also serve as a precursor of the pigment melanin and for the formation of Coenzyme Q10 used to shuttle electrons from cytochrome one to cytochrome 3.
The other chemicals in the matrix, like exotic atoms, can alter the dielectric values in the mitochondrial matrix because they change the overall charge inside the matrix. When we alter the dielectric values of tissues guess what interaction changes the most? Any change in a dielectric value directly alters the electromagnetic force generation capable between things in the water. It changes the scale of action. People forget that the electromagnetic force gets stronger as the scale of action gets smaller and vice versa. This changes the viscoelastic tensions in mitochondria and this changes how much redox power can be produced from our mitochondria.
CITES
https://www.ncbi.nlm.nih.gov/pubmed/7794222