
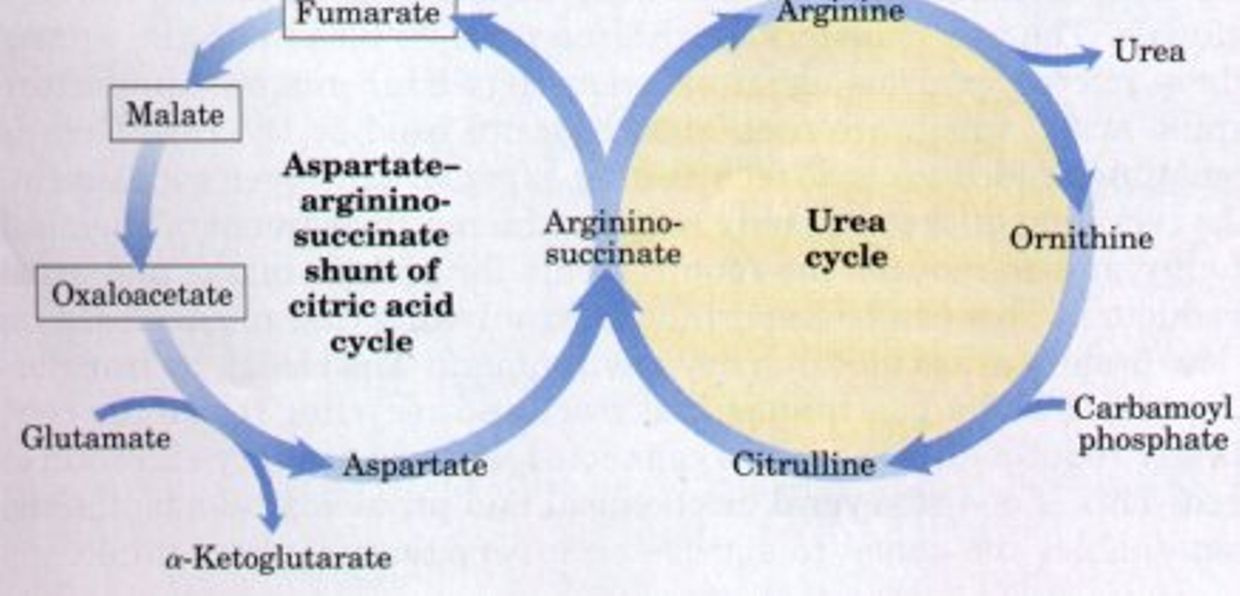
The “Krebs bicycle,” composed of the urea cycle on the right, which meshes with the aspartate-argininosuccinate shunt of the citric acid cycle on the left. Fumarate produced in the cytosol by argininosuccinate lyase of the urea cycle enters the citric acid cycle in the mitochondrion and is converted in several steps to oxaloacetate. Oxaloacetate accepts an amino group from glutamate by transamination, and the aspartate thus formed leaves the mitochondrion and donates its amino group to the urea cycle in the argininosuccinate synthetase reaction. Intermediates in the citric acid cycle are boxed.
The oxidation of acetyl-CoA to CO2by the TCA1 cycle is the central process in energy metabolism. However, the TCA cycle also functions in biosynthetic pathways in which intermediates leave the cycle to be converted primarily to glucose, fatty acids, or non-essential amino acids. If TCA cycle anions are removed from the cycle they must be replaced to permit its continued function. This process is termed anaplerosis.
Because the TCA cycle cannot fully oxidize 4- and 5-carbon compounds, these intermediates must be removed from the cycle by a process termed cataplerosis. Cataplerosis may be linked to biosynthetic processes such as gluconeogenesis in the liver and kidney cortex, fatty acid synthesis in the liver, and glyceroneogenesis in adipose tissue.
Cataplerotic enzymes present in many mammalian tissues include P-enolpyruvate carboxykinase (PEPCK), glutamate dehydrogenase, aspartate aminotransferase, and citrate lyase.
Instead of giving you a boring biochemistry lesson I am going to use a hormone that most of you know to help explain how the anaplerosis/cataplerosis cycles in the TCA cycle affect the urea cycle and can later the methionine cycles to help explain many things about life you might not have realized are going on. You really do not need to understand the steps you just need to understand the overal effects to understand your life.
Let me show you how testosterone, cell growth, all link to the sun cycle diurnally via the mitochondria to explain the TCA/urea cycle in a larger context.
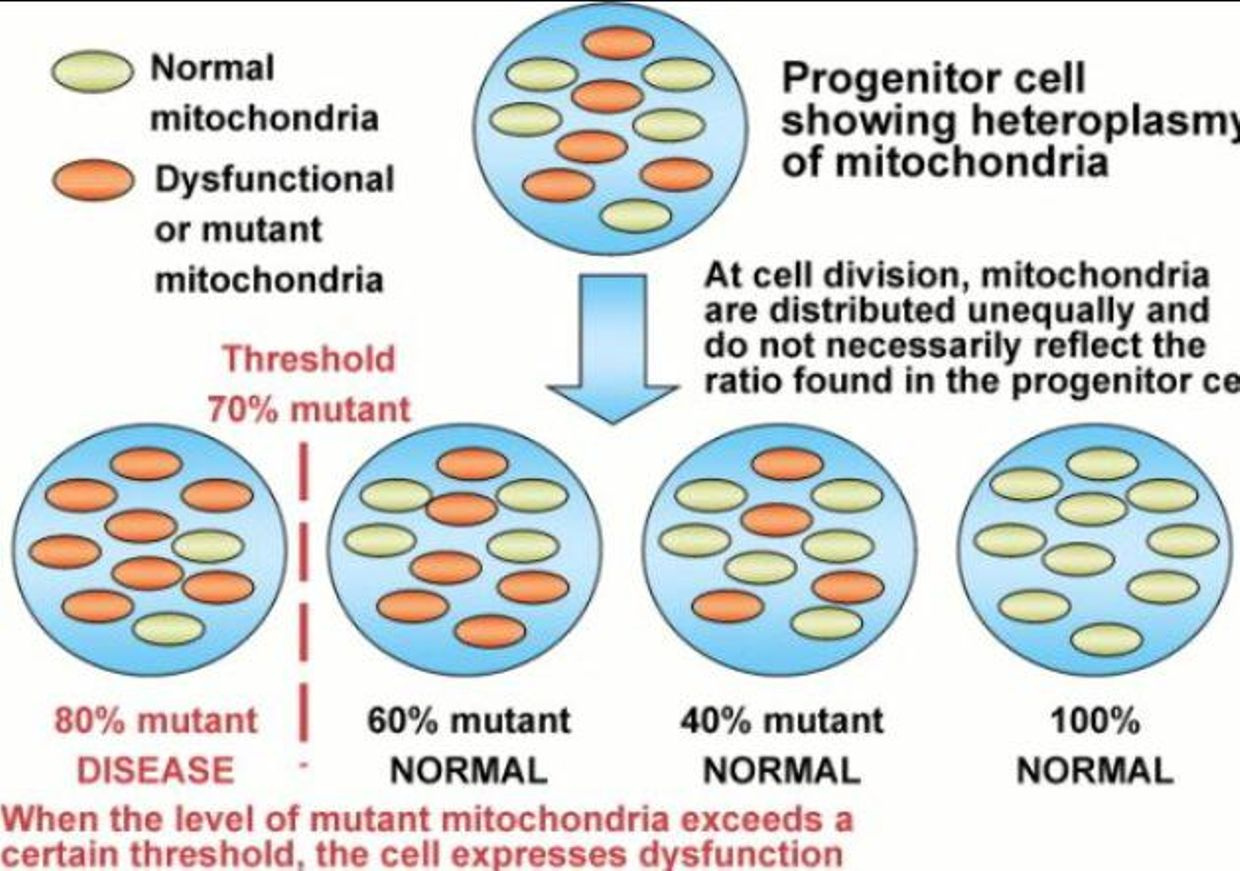
As we age does testosterone drops in both sexes to protect us from mitochondrial diseases which spike as we age and heteroplasmy rises. This rise in heteroplasmy is a maker for poor autophagy and apoptosis in mitochondria. While it’s good to have a decent immune response to pathogens, an overreaction to them — as occurs in highly virulent influenza strains, SARS, dengue and many other diseases — the repsonse to pathogens can be more damaging than the pathogen itself depending upon the state of your inner mitochondrial membrane. This shocks many people when they hear it for the first time.
This actually occured in the 1918 flu epidemic in humans. Those with the strongest immune systems tended to die more rapidly and more consistently (men). Women, children and the elderly had much better survival than young men. Why did this happen? In 1918 most young healthy men were working inside of factories under new electric lights and not in the sun.
What people have forgotten is that chronic exposure of the skin to UV light tends to limit the production of sex steroid hormones in our eyes and skin by way of our blood plasma. So men with high solar exposure should be expected to have lower sex steroid hormones. Sex steroid hormones are progrowth and facilitate biosynthesis from the TCA and urea cycles if Vitamin D levels are low. If Vitamin D levels are high it puts a cap on biosynthesis and growth via the cell cycle. It turns our Vitamin D3 and the VDR receptor curb cell growth directly on the inner mitochondrial membrane via two processes call anaplerosis and cataplerosis.
We also know that we need more sunlight as we age and we know that all cause mortality is reduced by sunlight. This hints to us that exogenous testosterone might be something we need to be careful with as we age or as our Vitamin D levels drop for any reason. I do not believe exogenous testosterone use is always bad to use as one ages but it must be put in context of the persons haplotype, SNP/SAP’s and their current environmental choices. Sunlight exposure of the skin is one of the major factors I look at.
Women have to pass epigenetic information to children which men do not have to do so, a woman’s immune system is built to be more sensitive and specific to program their offspring. This is also why women tend to get more autoimmune conditions as well. When they are in environments that cause immune activation from electropollution we should expect more Autoimmune conditions to manifest in them when Vitamin D3 levels drop. This facilitates a progrowth bias in their immune systems with poor controls.
We should also realize because of these relationships that solar exposure of the skin and eye are nature’s best vaccine for prevention of these conditions but few people really understand how the pieces fit in humans because they do not understand light of the sun well on the inner mitochondrial membrane.

In fact, the rapid, nongenomic effects of vitamin D3 appear to be mediated by the VDR directly on the inner mitochondrial membrane. The key activity of the Vitamin D receptor is to inhibit ECT to slow electron flow on this membrane. This is the breaking mechanism fine tunes apoptosis efficiency to keep a cap on uncontrolled growth in tissues with high oxygen tensions who use the TCA cycle for biosynthesis. As the VDR receptor lowers ECT flow, the distal ATPAse can be augmented by the 42% of red light in the sun to still affect the spin of the ATPase Fo head and affect the 4 red light chromophores on cytochrome c oxidase.
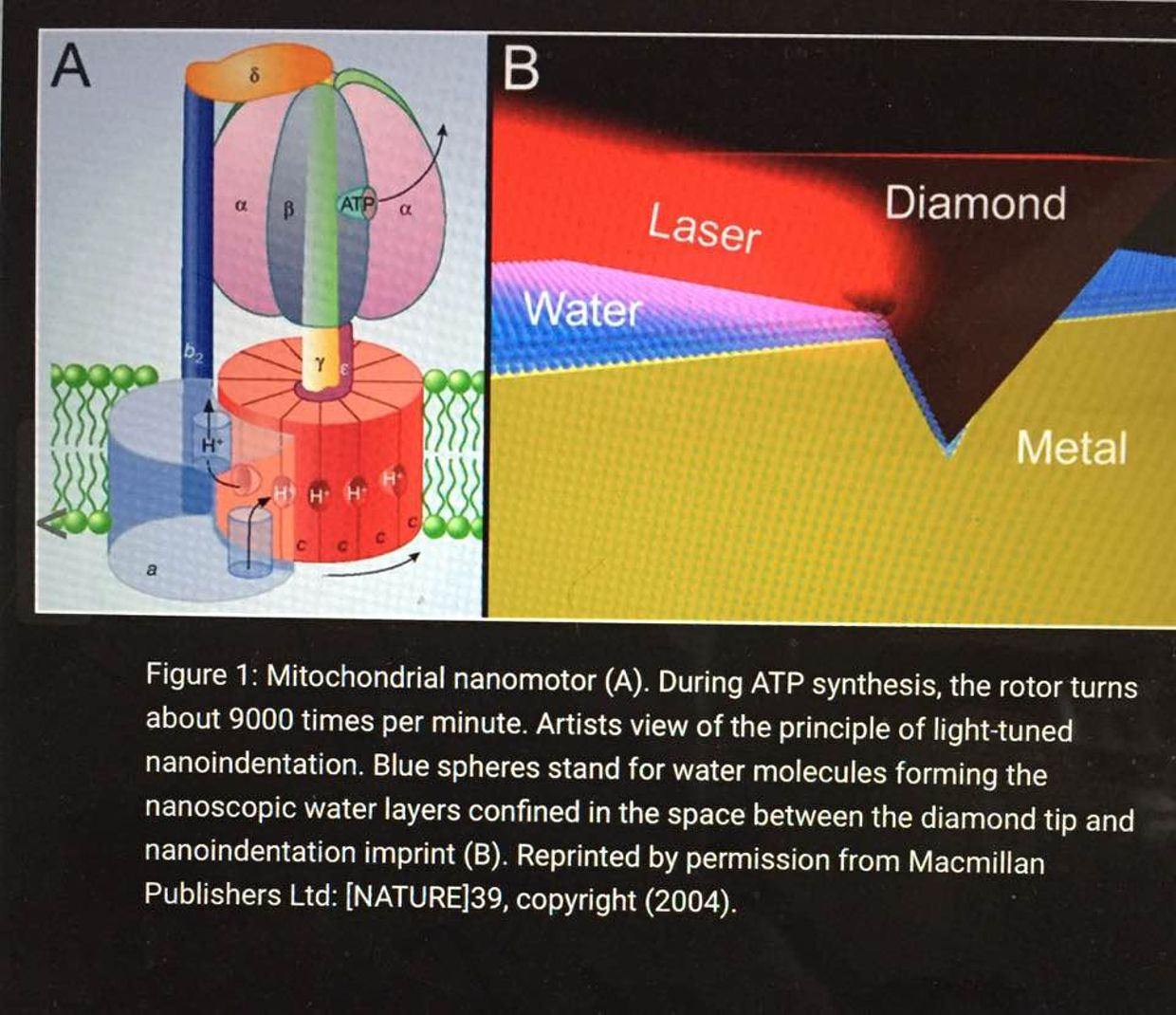
The inner mitochondrial membrane contains many copies of a protein called the F0-F1 ATPase. This is also called ATP synthase. It consists of two parts: the Fo component spans the membrane and provides a channel for protons to move into the matrix from the intermembrane space. The F1 component is a complex of five proteins with the composition α3β3γδε, with a molecular weight of about 360,000. This remarkable complex couples movement of H+ to the synthesis of ATP.
The ECT pumps H+ ions out of the matrix into the intermembrane space. Because the H+ ions move without counter ions, this movement is an outward current that separates charge, and therefore there is a potential developed across the inner mitochondrial membrane. This potential varies depending on the state of mitochondrial activity, but a typical value is about 160 mV, negative inside. In addition to the potential, there is a concentration difference in H+ established across the membrane. The pH of the intermembrane space is about 7.0, whereas the pH of the matrix is about 8.0. The pH differences also has huge implications on favoring certain isoforms of hydrogen. Consider the following example to improve your understanding of these nuances.
The pH at room temperature is ~7 meaning there are about 10^-7 moles of H+ per liter of water at room temperature. As the temperature increases, the ability of water to ionize in this way INCREASES and so the concentration of H+ in an aqueous solution in a cell will ALSO increase and hence the pH will drop.
The physical chemistry here all favors the formation of H+ bonds over deuterium bonds in an aqueous heat bath we call a cell. This is called the chiral effect. You’ll be getting a full blog on that topic soon enough.
Just how big a deal is it when you are a true blue mitochondriac? In all cases, raising the temperature, invokes thermal vibrational and entropic effects in all proteins, lipids, and carbohydrates in a cell. This tends to preferentially stabilize H+ over D bonds in mitochondria. This implies that heating favors H+. This is why mammals release heat from the mitochondria. Any place a mitochondria is located in a mammal they want to keep deuterium concentrations low. This chiral heating effect keeps deuterium free to roam in the ECF and blood plasma so it can perform its duties not confined to the boudaries of the kinetic isotope effect. What cells dominates the blood plasma? An RBC. RBC’s have no mitochondria.

The link between the VDR receptor and NO production is very strong in the skin and the eye. Many steroid receptors and nuclear transcription factors enter the mitochondrial compartment, where they either exert transcriptional regulation of mitochondrial DNA or control mitochondrial biogenesis and metabolism. These facts show why I staunchly believe that sunlight is nature’s vaccine for most mitochondrial diseases. Based on my reading of the literature and observations in hacks, I have concluded that the VDR is highly protective against mitochondrial disease from high heteroplasmy rates. THe VDR needs high levels of sulfated Vitamin D3 to operate properly.
When Vitamin D3 and the VDR bind some amazing things occur inside of your mitochondria.
Mitochondria need less electrons from food substrates because the function of Vitamin D3 bonding to the VDR restrains mitochondrial respiratory activity, and this lowers ROS and RNS. This occurs independent of any other factor in mammals.
But the key feature is that the VDR receptor is the main regulator or carburator that allows the cell to spare metabolic intermediates from the TCA and urea cycle. Anaplerosis is the act of replenishing TCA cycle intermediates and cataplerosis is the term used for removal of TCA intermediates for biosynthesis. The VDR receptor inhibits cataplerosis and this should make sense to you now. As you remove substrate from the main beta oxidative pathways you need to control the flux of electron flow in the inner mitochondrial membrane to put a cap on ROS/RNS and oxygen needs.
Cataplerosis removes intermediates for biosynthesis in normal growth to which may be diverted from oxidative metabolism toward a biosynthetic fate, supporting cell growth. This process has no brake in cancer because the VDR receptor only operates with a base level of sulfated Vitamin D3. Without it, the VDR receptor is inactivated and this single factor alone supports high ECT, with inactivation of apoptosis. It also allows the chronic removal of TCA and urea cycle intermediate to support uncontrolled growth. This is what we see in cancers, autoimmune conditions, and obesity.
Cataplerotic reactions effectively allow your cells to steal the 4-carbon intermediates from the TCA for biosynthesis to support growth.
REMEMBER RBC’s HAVE NO MITOCHONDRIA. WHAT ELSE MAKES THEM SPECIAL?
RBC’s use a Warburg metabolism. Yes, you heard that correctly. Most people associate that metabolism with cancer but it comes in two versions. Pathologic and non pathologic.
Cells with non pathologic Warburg metabolisms use gluconeogenesis. Gluconeogenesis is an overall cataplerotic reaction because it steals oxaloacetate to eventually form glucose. RBC’s, Muller cells, oligodendroglia cells, and embryonic stem cells use gluconeogenesis. Oligodendroglia cells make myelin in our brain. The non pathologic version occurs when oxygen levels are lowered and controlled this and it limits net cell growth.
When oxygen is raised in these cells this supports strong cell growth that can be uncontrolled. In the case of stem cells, you need to keep oxygen levels low to keep them quiescent until we need them. This means ESC cannot and should not use TCA/urea cycles to exist. In fact. ESC have immature mitochondria that cannot use the TCA or urea cycle by design. They have to rely on the older evolutionary pathways of glycolysis and the PPP to operate when the body needs them to jump into action.
WHAT HAPPENS IN THE TCA CYCLE DURING BIOSYNTHESIS?
Aspartate biosynthesis also requires oxaloacetate, so when aspartate is synthesized, the result is less net oxaloacetate. When cataplerotic reactions occur (in terms of TCA), there is less oxaloacetate to condense with acetyl CoA. This is why proliferative retinal disorders show up in diabetics with obesity and low Vitamin D levels. It is also why AI’s spike with low D3 levels. It is also why brain tumors and breast cancer spike with low vitamin D levels. Sunlight is the brake for cataplerosis in cells who use the TCA or urea cycles. That brake requires a MINIMAL level of Vitamin D3 production to control unfettered growth to bond with the VDR to slow ECT flow and increase apoptosis. Now think about what I told you in the May 2018 webinar carefully.
Women, with their more robust immune responses, are twice as susceptible as men to death from the systemic inflammatory overdrive called sepsis. This is something to keep your eyes on as 5G takes over because we should see more women die from sepsis in big cities if these links to the immune system hold true. Men are designed to have a slightly weakened immune system than women to balance growth and cataplerosis carefully. A slightly weakened immune system seems to favor anaplerosis over cataplerosis in the TCA cycle. This curbs unfettered growth assuming the VDR receptor is activated. This might be why men have more body hair than women and it is likely why women have less on their skin. Hair on the genital region would also lower solar exposure to support growth of the germline in both sexes via the Viamin D and VDR mechanism. Strong sunlight tends to lower fertility. Strong blue light toxicity does the same.
So perhaps having a somewhat weakened (but not too weak) immune system can prove more lifesaving than life-threatening for a dominant male in the prime of life. UV light increases your immune system, and testosterone seems to lower it. I believe it is designed to be dynamic to react to the EMF’s in our environment. From an evolutionary standpoint the main EMF mitochondrial are built to work with are sunlight but today this is no longer true. The fact that sepsis now kills 18 million people per year in a 4G world these links are incredibly important to follow to gain insight on how we should handle sex steroid hormones as we age in a 5G world. I have a sense the answers won’t be easy because the equation requires nonlinear thinking.
CITES:
https://www.sciencedirect.com/science/article/pii/B9780123821638000219