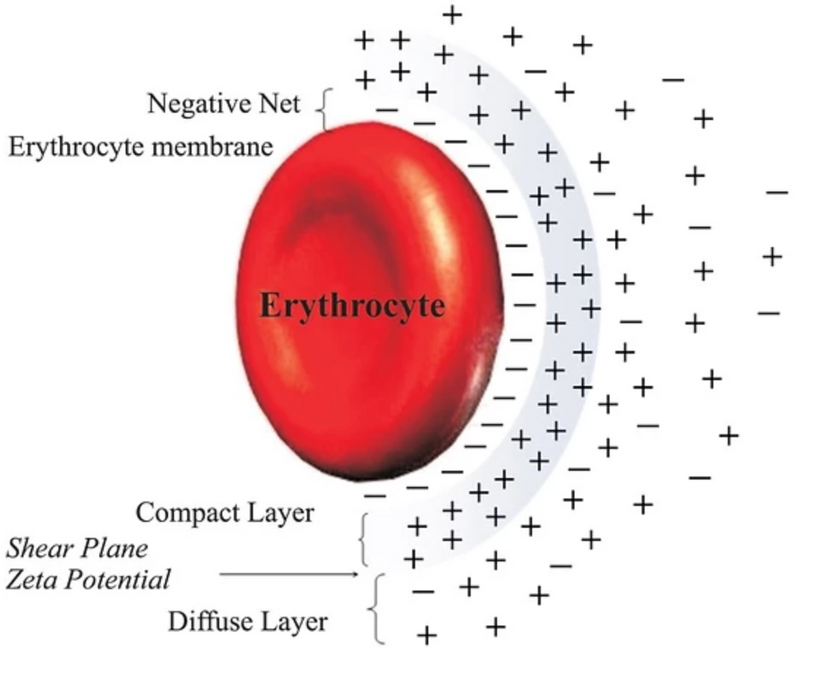
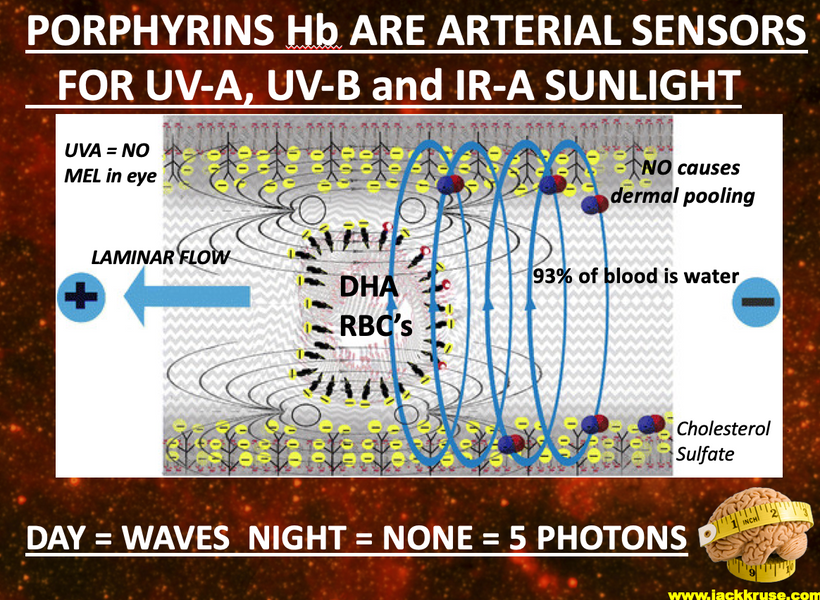
If you have followed the last four blogs what you are about to read might not shock you. If you have not, this blog will shock you and it is a signal that you better go re-read the last three blogs carefully.
MAMMALS INNOVATED METASTASIS TO SURVIVE THE KT EVENT
The essence of the KT event was that an asteroid blew out a 50-mile cube of Earth into space and blocked photosynthesis for some time afterward. This immediately cooled the planet by blocking sunlight and making it very cold and it disrupted the food webs on Earth killing all the animals who depended upon it and could not adapt to this rapidly changed Earth.
65 million years ago mammals were animals that had been on Earth for 150 million prior to this event, but they mainly used melanin in their exteriors to help them survive underground. That included their hair and skin. Melanin is well known to also affect animals feeding behavior and appetite. These mammals were small and did not have significant neural computation power during the reign of the dinosaurs. Since modern birds can see the UV spectrum, and modern birds are direct descendants of therapod dinosaurs it was very likely that all dinosaurs could see UV light in their retinas. This would have made early mammals easy prey for the larger dinosaurs and this relegated the mammals to an underground existence.
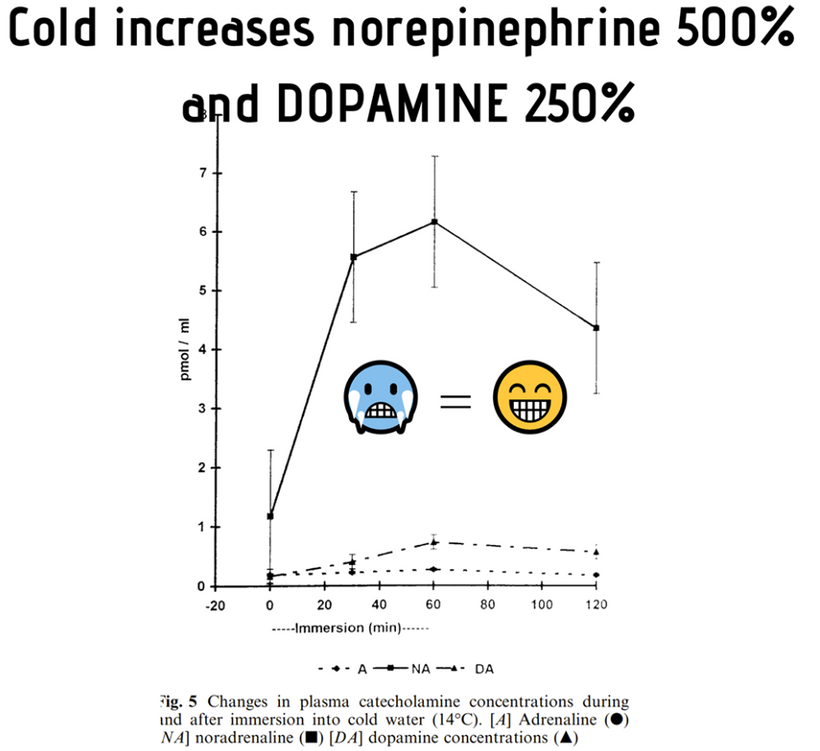
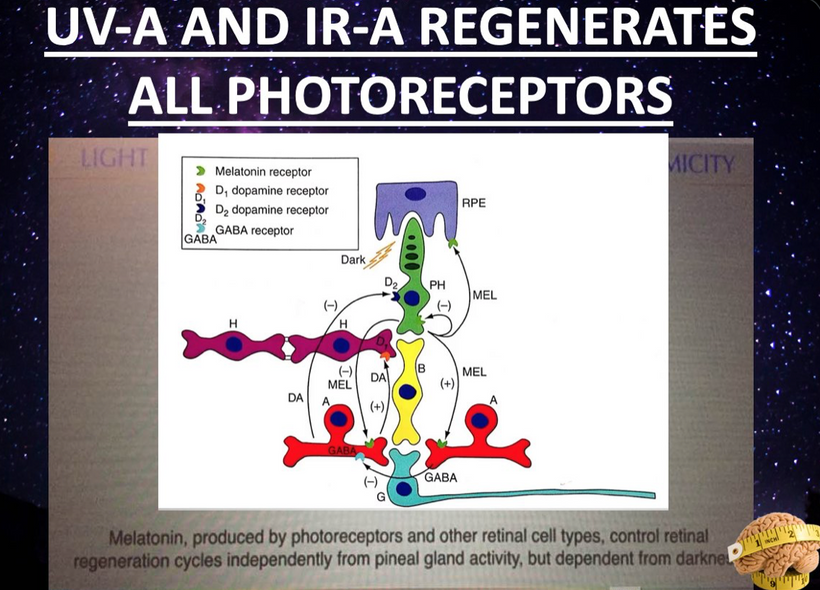
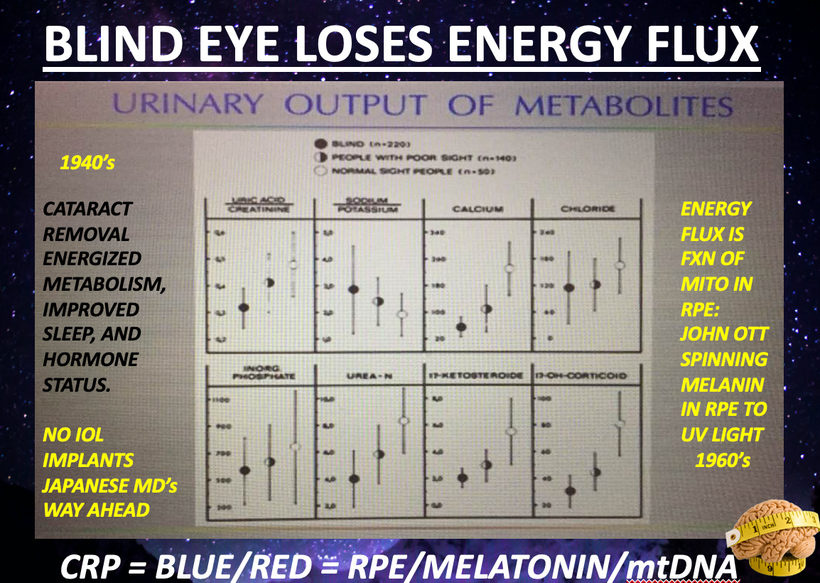
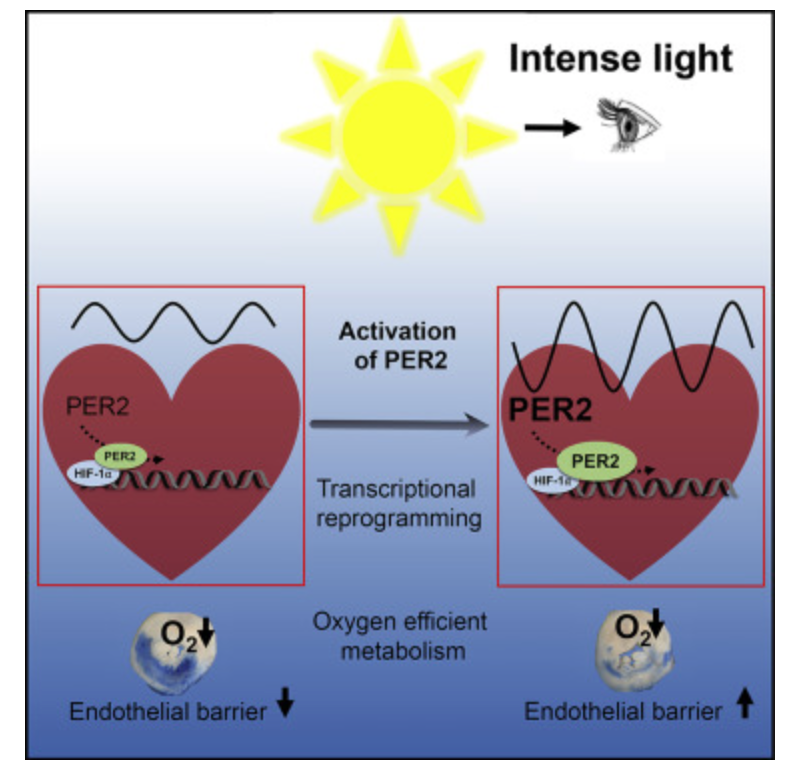
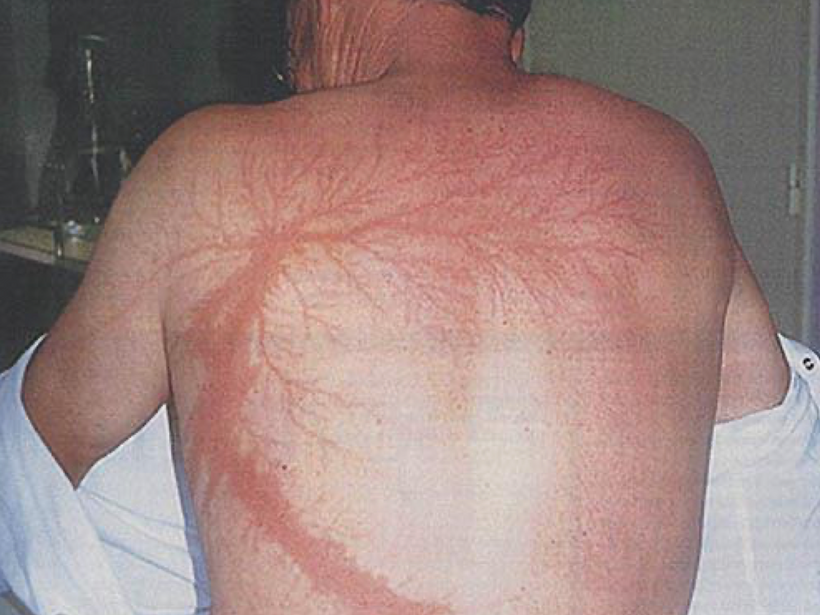
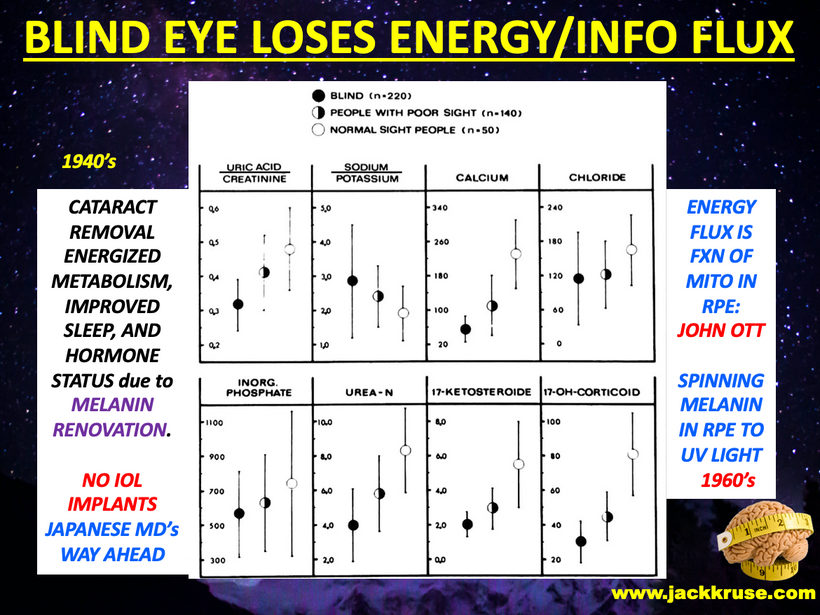
When a mammal’s exterior is “light stressed” caused by any lack of UV light, they begin to move its melanin along its neuroaxis. We call this neuroplasticity today. Recall that melanin is a neuroectodermal structure and it is abundant in the skin, hair, and brains of mammals. This is precisely what the KT asteroid that hit the Yucatan peninsula was 65 million years ago. UV light likely disappeared from the surface of the Earth for many years. How many years is unknown but I bet it was on the order of a human lifespan or shorter. Why do I make this guess? The picture below is why I said it to Dr. Huberman. I was able to reverse this process in 3 months. The speed shocked me and so did a return of hair color.
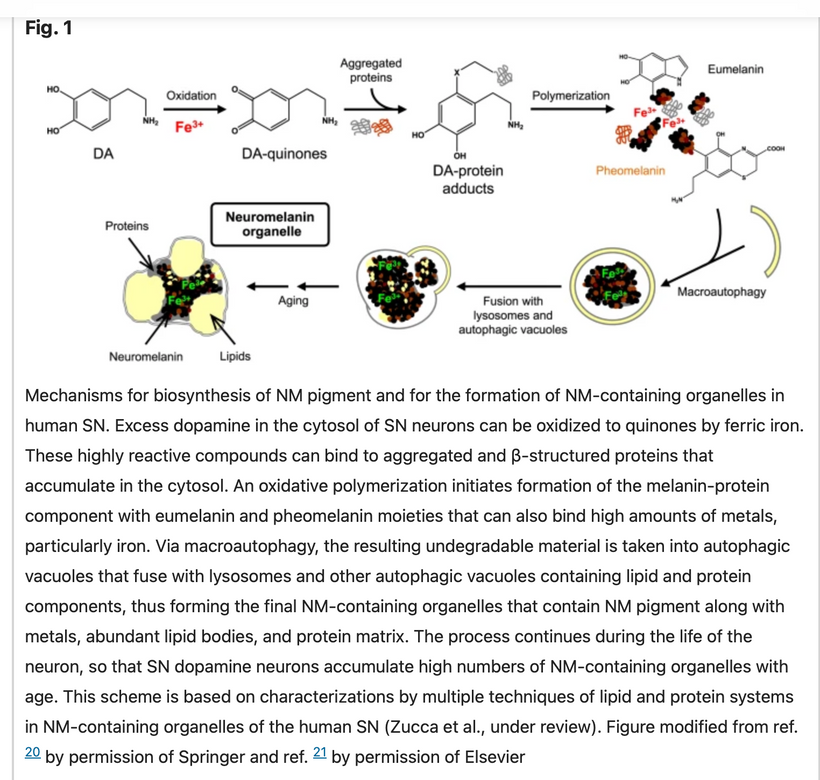
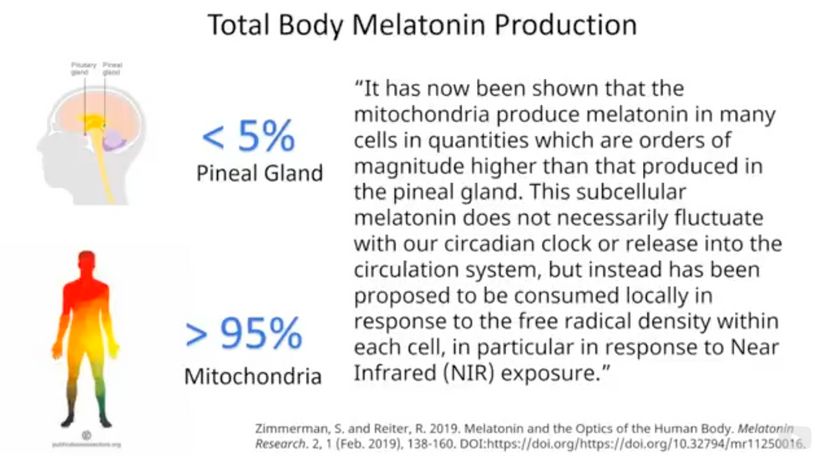
When mammals lost their normal solar UV signal they also lost the ability of their melanocytes to undergo mitosis. This is covered early in the book below in the picture of experiments done on onion roots. I led the Huberman/Rubin/Kruse podcast off with this fact that is not well-known in centralized science. HYPERLINK It is well-known in the biophysical literature. This was key in understanding how melanin could move so fast in mammals.
In fact, every living cell has been found to emit ultraweak-UV light. What we do not know today is the spectrum of that light or how it varies with metabolism or in cell lines.

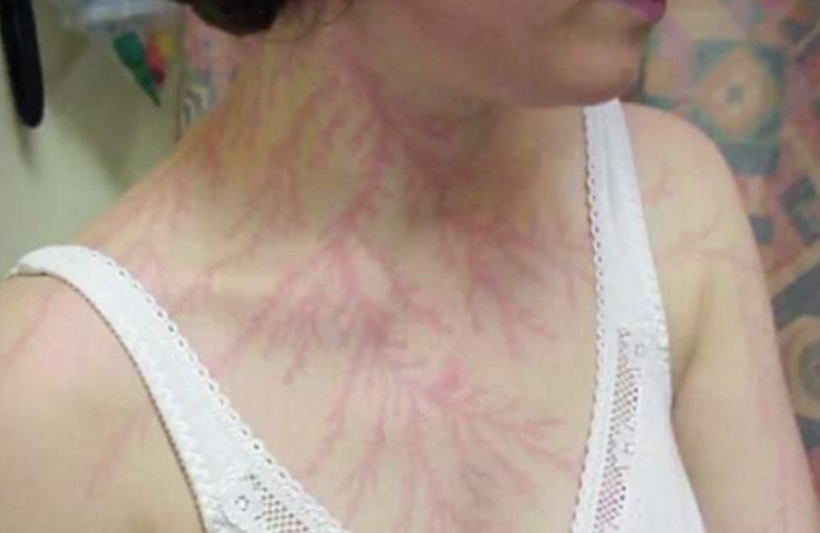
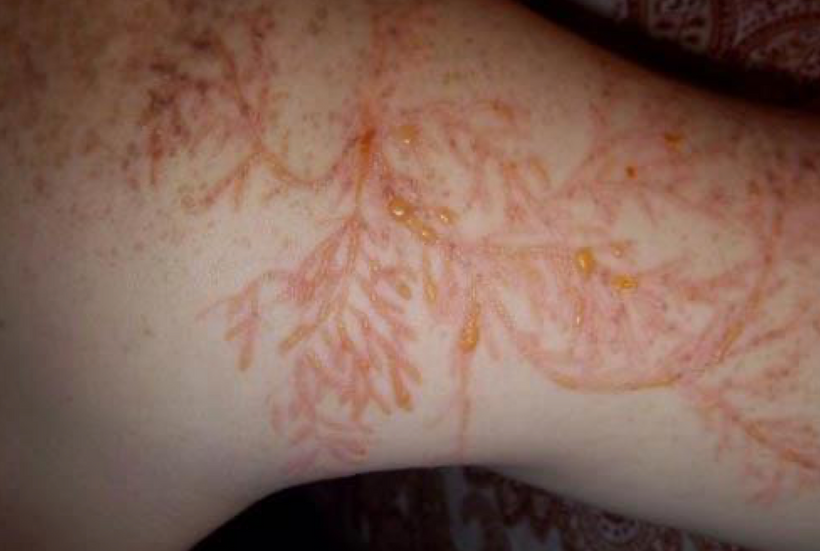
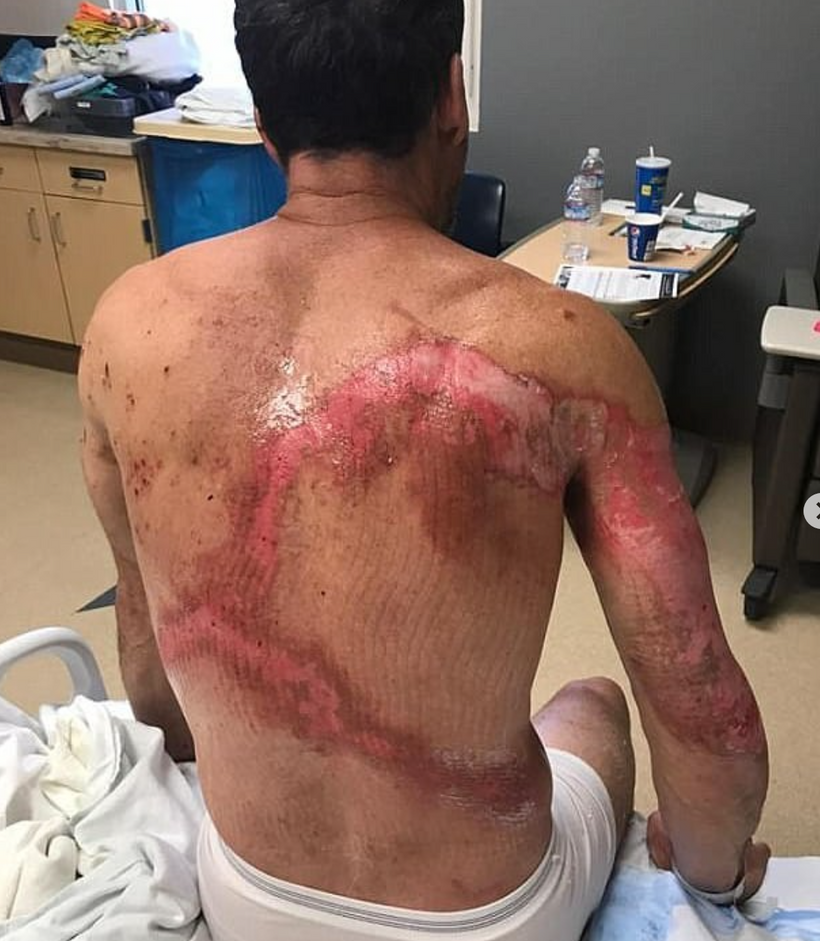
The dermatologist who knew about the patient’s treatment above with me called me up to discuss the details of the reversal of melanin in this case and what my theories were about the outcome on her skin and hair. During our discussion, she asked me did I know about “Marie Antoinette Syndrome.” I had never heard of it in 2009. She thought based on my speculations that I might be able to explain this phenomenon for her. I was stunned when I read about it. (below)
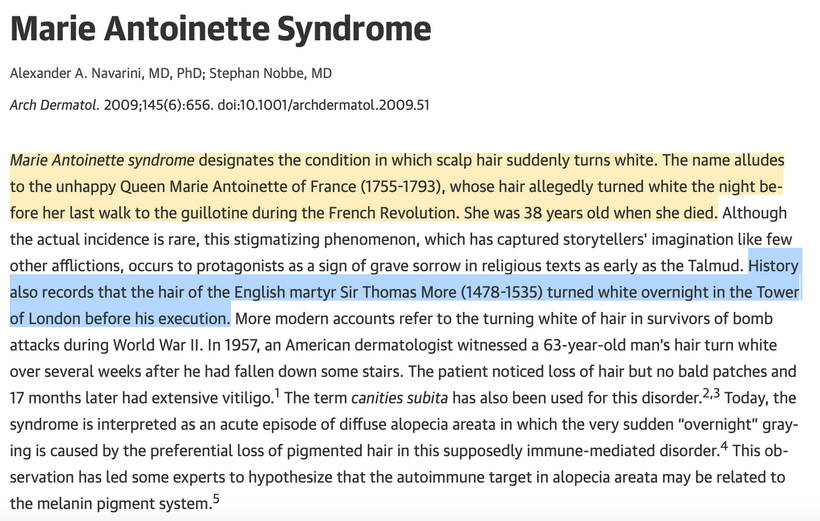
I found out in researching melanin movements from hair that extreme rapid stress of any type can affect mammals and turn hair white overnight (or at least very quickly). This appears to have happened to Marie Antoinette (above) before the morning she walked to the guillotine. Sir Thomas More’s hair is also said to have turned white overnight in the Tower of London before his execution.

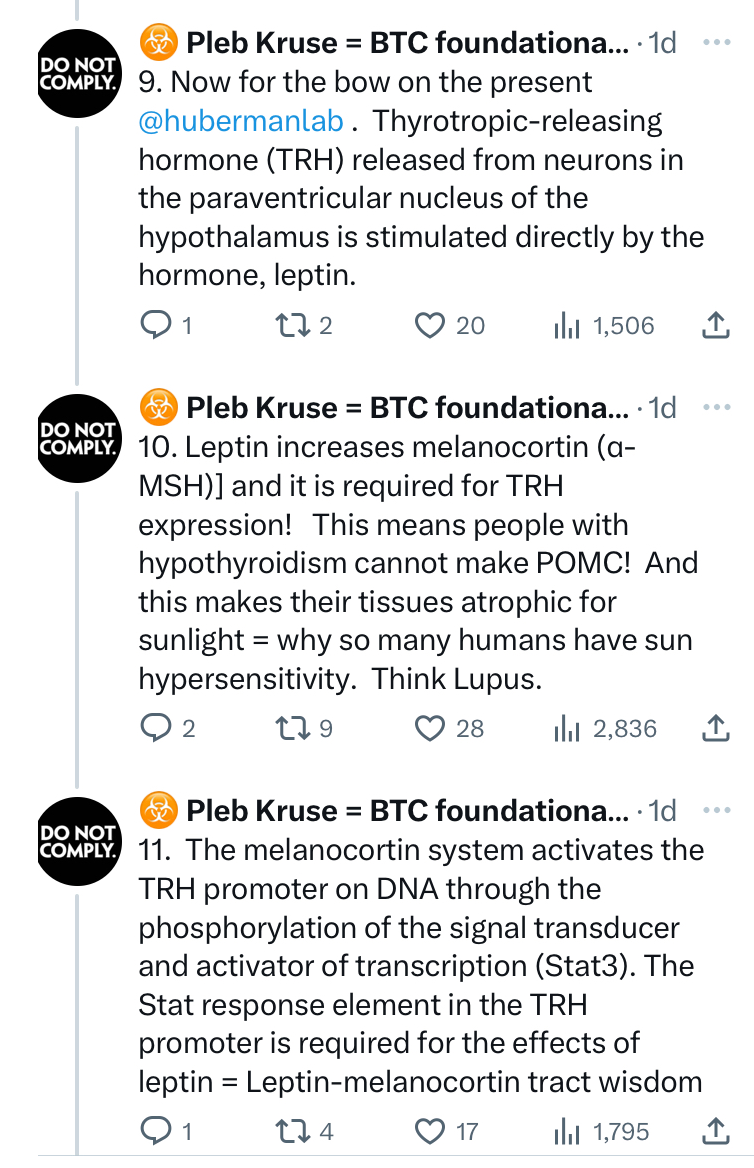
^^^^This told me changes in melanin can be rapid during a light-stress event.
WHAT ELSE DO WE KNOW TO BE TRUE TODAY?
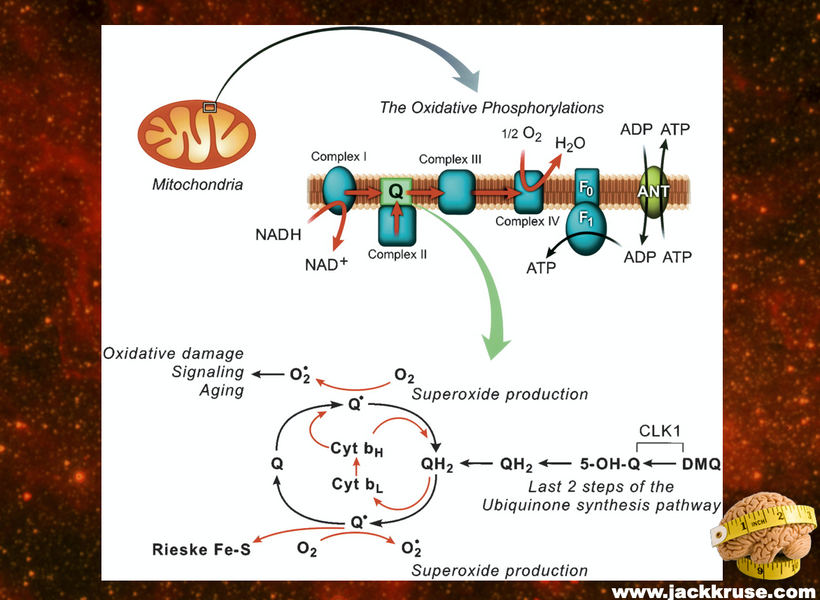
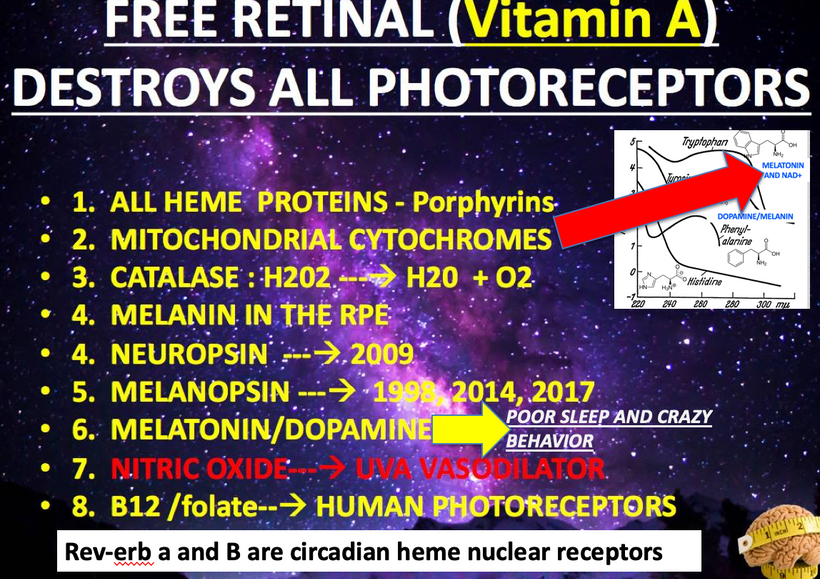
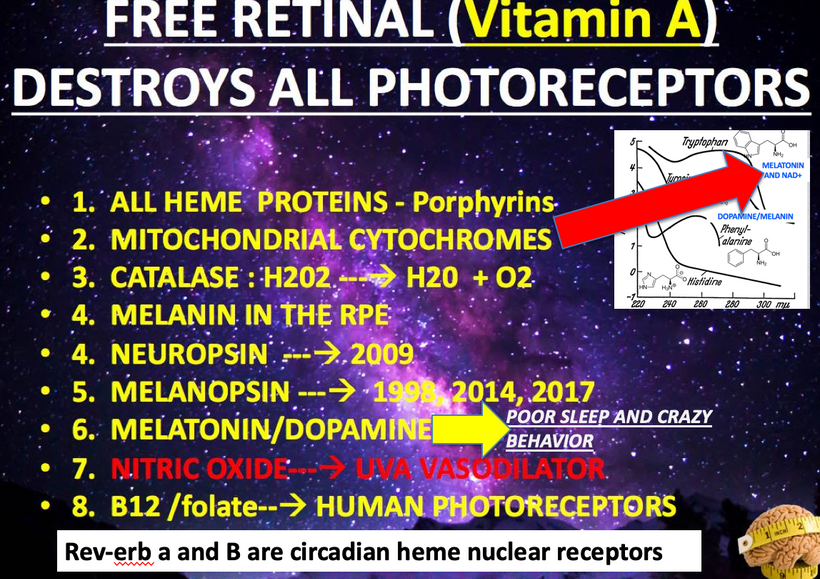
Mitogen-activated protein kinases (MAPKs) are serine and threonine protein kinases that are highly conserved in ALL eukaryotes and are involved in signal transduction pathways that modulate physiological and pathophysiological cell responses. Mitosis in all cells is controlled by Ultraweak UV light release from cells as we learned from Gurwitsch’s work with onions in 1923. This was confirmed by Fritz Popp in the 1960s. (pic below)
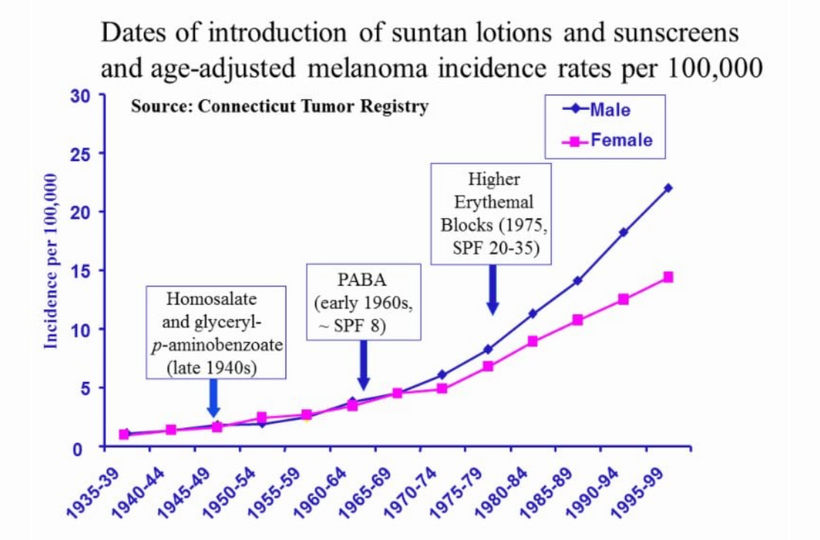
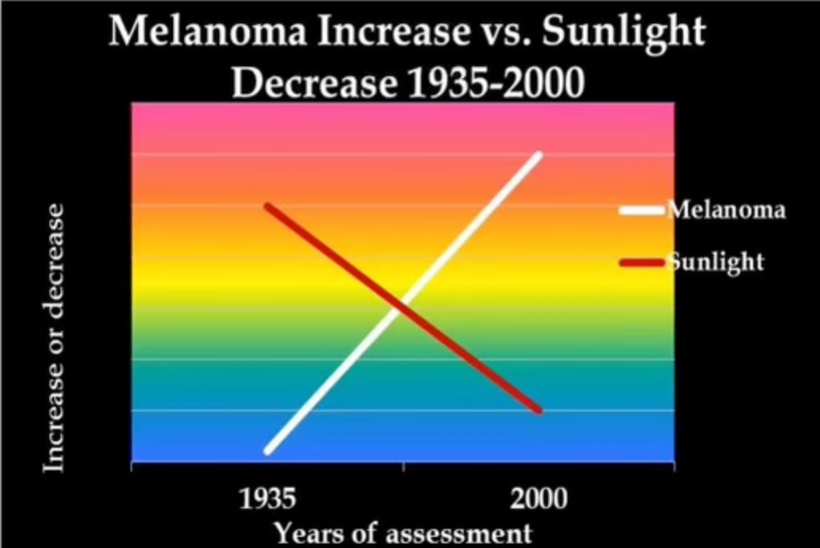
When cells become unable to create this light they lose the ability to undergo mitosis and this allows the cell to become mobile and roam the body until they find the UV light signal they look for in their environment. Tissue devoid of the ability to make VUV light endogenously is the sine qua non of a low redox state in local mitochondria. This allows them to continue to move toward the surface until UV light is found in the environment Once they find it they stop moving and begin to re-enter the cell cycle and grow again. I no longer think melanoma is what modern dermatology thinks it is. It is a sign of horrendously poor redox at a mitochondrial level. This implies blocking the sun for any reason sets the stage for melanoma. Is there any data to support this idea? (below)
When VUV-IR-A light is lost locally at the tissue level cell can move until they find adjacent cells that can create the VUV-IRA light and then reenter the cell cycle to cause abnormal growth = Cancer.
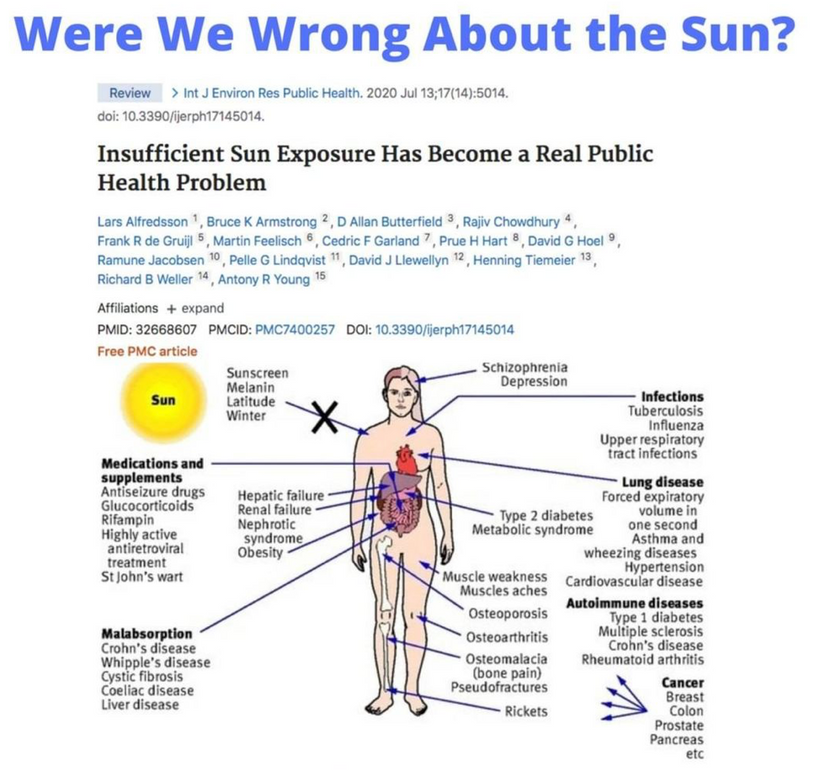
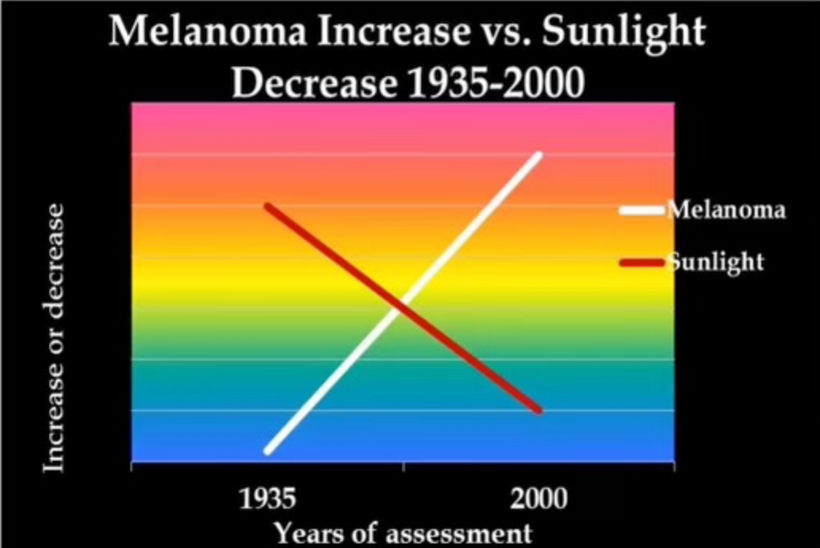
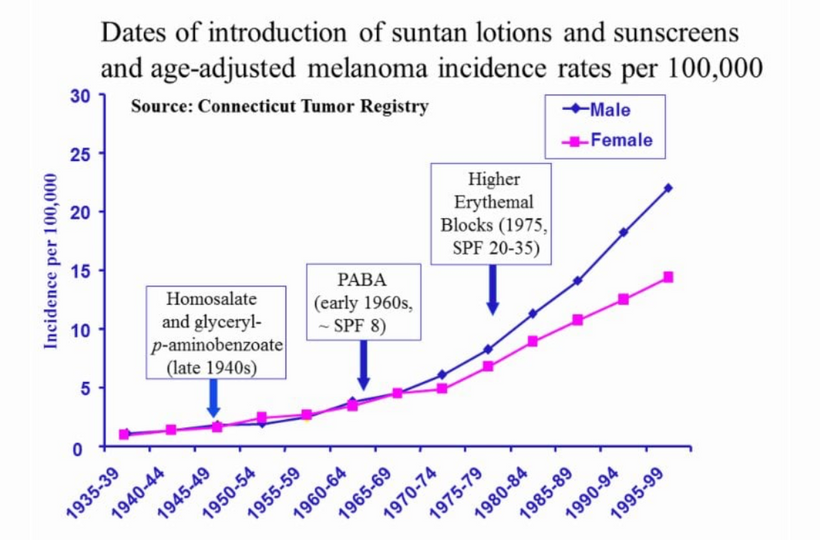
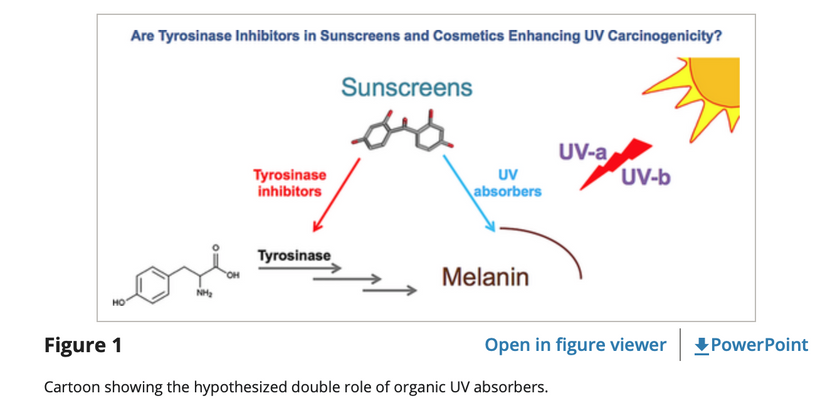
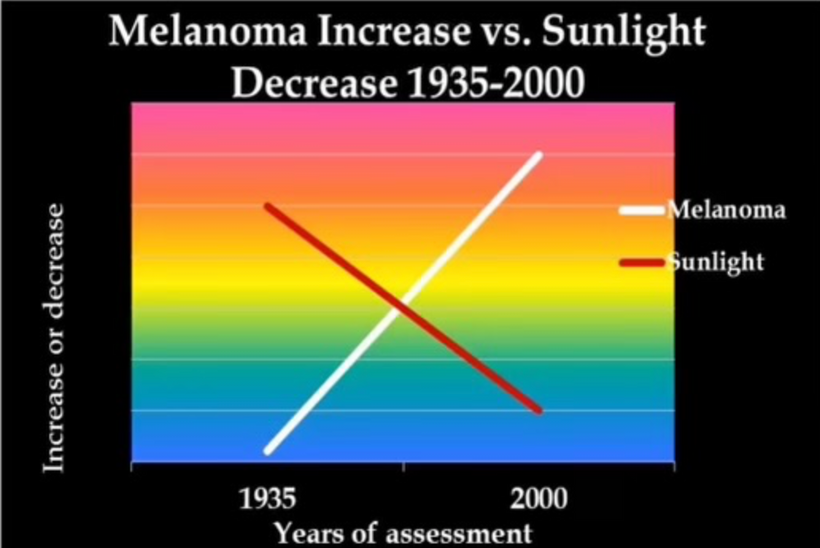
Don’t you find it amazing that melanoma is rising when sunscreen use is rising, and people are staying indoors in fake light while everyone is being told the sun is toxic? Might modern centralized medicine be wrong about the sun?
I ASKED RICK AND HUBERMAN, “DOES NATURE REALLY MAKE MISTAKES?”
Listen to their responses when the podcast goes live.
Huberman was very shocked by my melanin thesis that sits below the Leptin Rx because he knows neurobiologists have reported that human melanin and amphibian melanin seem to be identical and their receptor pathways and actions are almost identical in human neuroectodermal tissues.
What Dr. Huberman did not know during our epic podcast is that melanin was linked to the early vertebrate tree by MCH = Melanin-concentrating hormone. He also did not know that cells retain their mobility when mitosis is blocked by a lack of UV light. He never heard of the onion root experiment which proved that UV light was the mitogenic radiation cells use to divide.
When UV light is absent in the environment, the activity around the cleavage products of POMC is altered due to the LIGHT STRESS.

Melanin is an ancient semiconductive protein in all life. Although the chronology of Melanin Concentrating Hormone discovery emphasized its early role in skin pigmentation, it is now believed that this function only emerged for the first time in evolutionary history by the last universal common ancestor of teleosts and holoceans, millions of years after the appearance of MCH synthesis in chordates (Kawauchi and Baker, 2004).
Vertebrates comprise all animal taxa within the subphylumVertebrata (chordates with backbones), including ALL mammals, birds, reptiles, amphibians, and fish. Vertebrates represent the overwhelming majority of the phylum Chordata, with currently about 69,963 species described. Human melanin has been linked to frog melanin by many researchers. Dr. Huberman mentioned them in our podcast so listen for it.

I linked the story together for him. This stunned him. Essentially melanocytes in humans have a lot in common with amphibians and this is because melanin is a wide band gap semiconductor that goes back directly to the Cambrian explosion 600 million years ago. (above) The same story is true for DHA use in life. Without DHA the central retinal pathways lose their semiconductive abilities. (below)

(MCH) is a cyclic peptide highly conserved in vertebrates and was originally identified as a skin-paling factor in Teleosts. In fishes, MCH also participates in the regulation of the stress response and feeding behavior. (below from Hollwich)
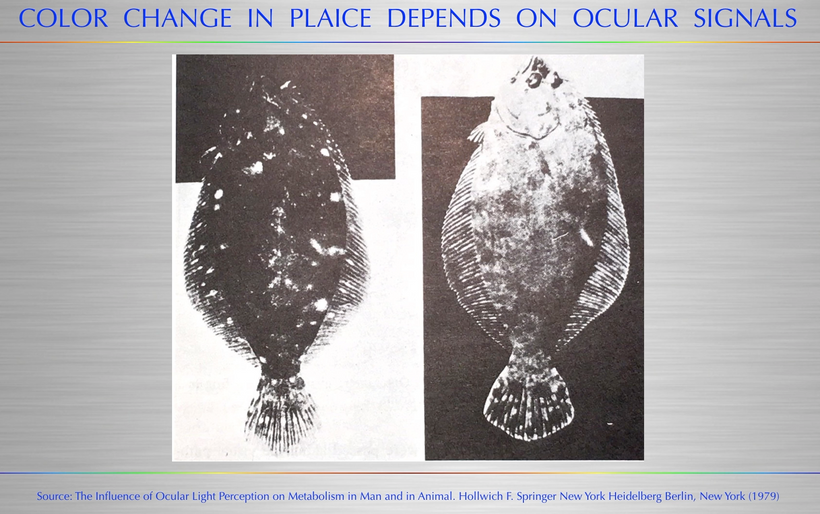
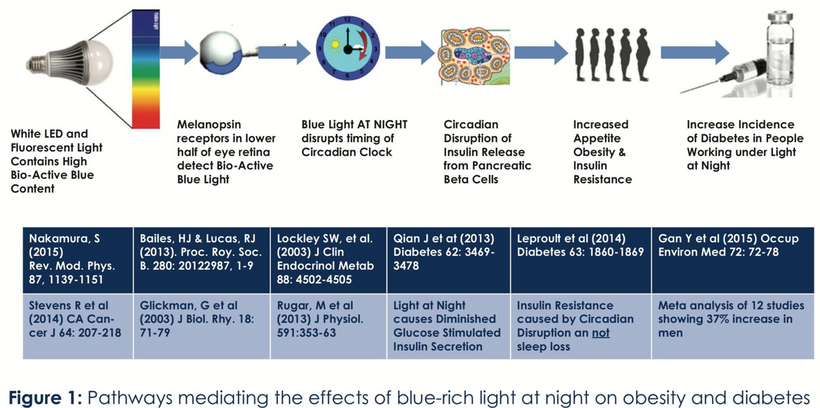
This is exactly what it does in mammals today as well. This is why modern humans are getting fat = leptin-melanocortin pathways have lost their melanin due to a lack of sun (Leptin Rx below). The KT event was the largest light stress test of the central MCH system in mammals in the history of their lifespan on Earth…….until the one we are living in now!!! Their response essentially guaranteed their survival when UV light was turned off by the asteroid.
Mammalian MCH is a hypothalamic neuropeptide with multiple functions, mostly controlling feeding behavior and energy homeostasis. Mammalian skin was critical in determining energy balance in them when photosynthesis was disrupted. In the 65 million years since this event leptin was innovated in mammals’ subcutaneous fat to link to the melanocortin pathways deep in the brain. Few seem to know that human adipose tissue expresses several melanogenesis-related genes. This told me that over 65 million years leptin replaced melatonin creation in mitochondria as the key energy accountant in mammals. It also explains why today in humans, circulating leptin levels are capable of changing hypothalamic POMC levels.
The presence of melanin in human adipose tissue was revealed both by Fontana-Masson staining and by permanganate degradation of melanin coupled with liquid chromatography/ultraviolet/mass spectrometry determination of the pyrrole-2,3,5-tricarboxylic acid (PTCA) derivative of melanin. HYPERLINK
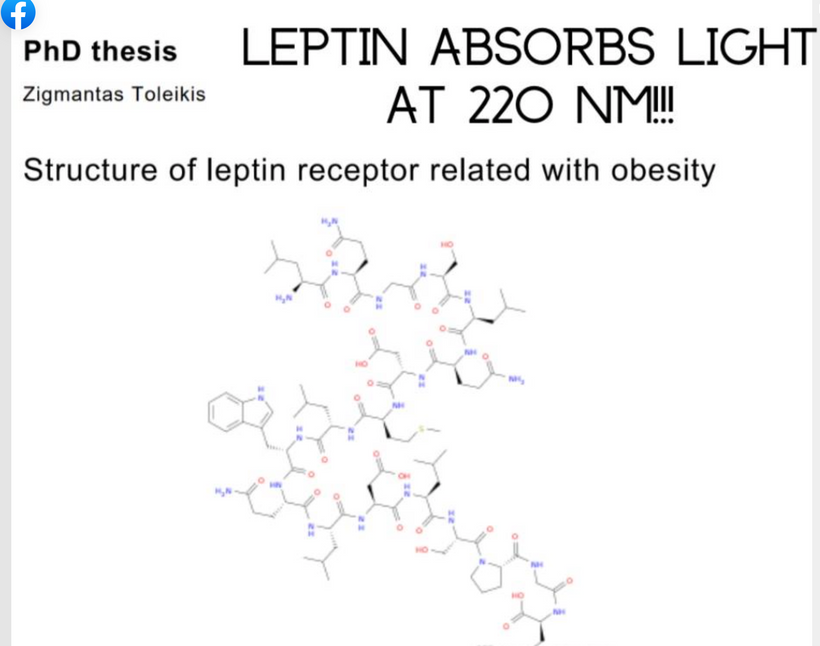
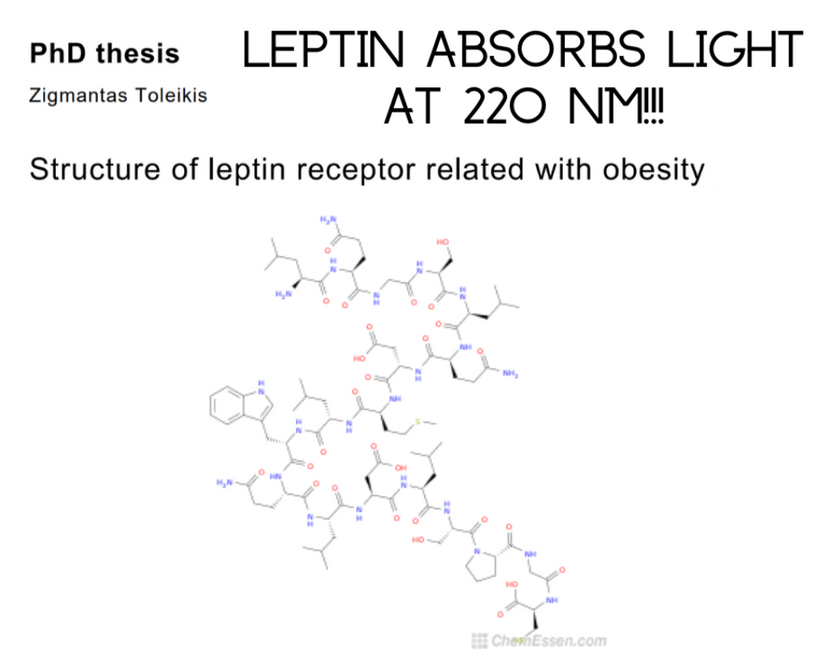
Leptin’s absorption spectrum is 220nm and in the deep UVC band. When I first learned this, it did not make any sense until I thought about Becker’s work on bone. This frequency of light is not provided by terrestrial sunlight. This caused me to look for ways we could generate UVC light inside of us. When I said this to Dr. Huberman he was very shocked. He told me later it was the biggest take-home from the light lessons I gave him in the podcast.
That is when I found deuterium in our blood and when I discovered the ability of wide-band semiconduction of producing more powerful light from the visible light the sun creates. In fact, 220 nm light corresponds to a band gap of 5.6 eV. This band gap is close to carbon’s band gap of collagen in cells as a semiconductor. I learned from Becker’s work on bone regeneration that collagen was an N-type semiconductor. It was then I found out carbon was a wide-band semiconductor that acts like a topologic insulator. This told me implicitly that leptin became much more important in the post-KT world in mammals for energy balance. It displaced melatonin’s critical importance in the hypothalamus. I believe this change was mediated by a melanin renovation using ferroelectricity to change our body plan.
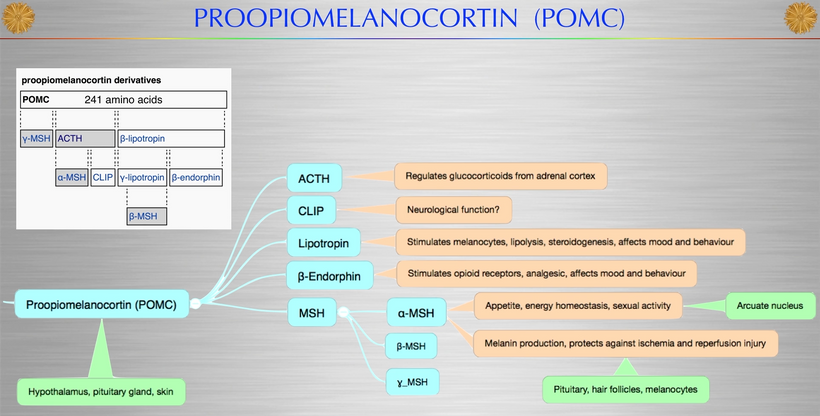
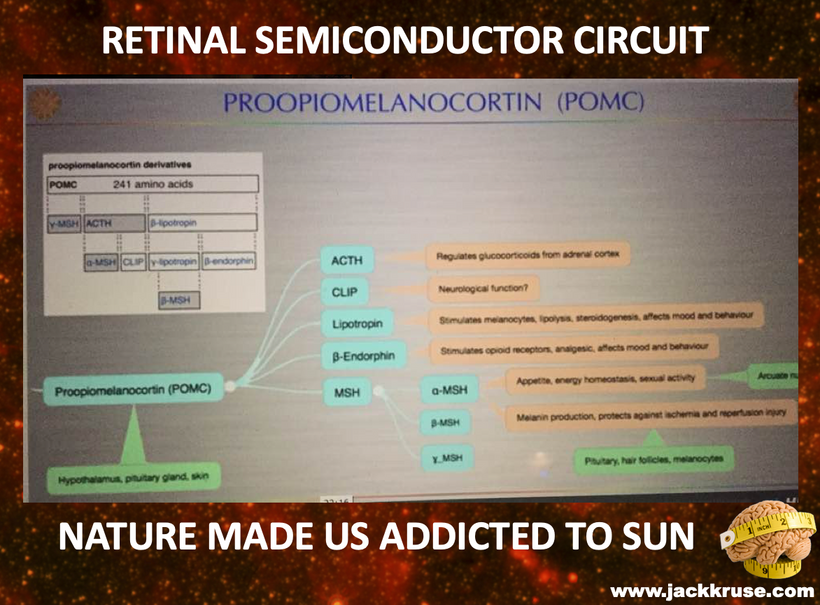
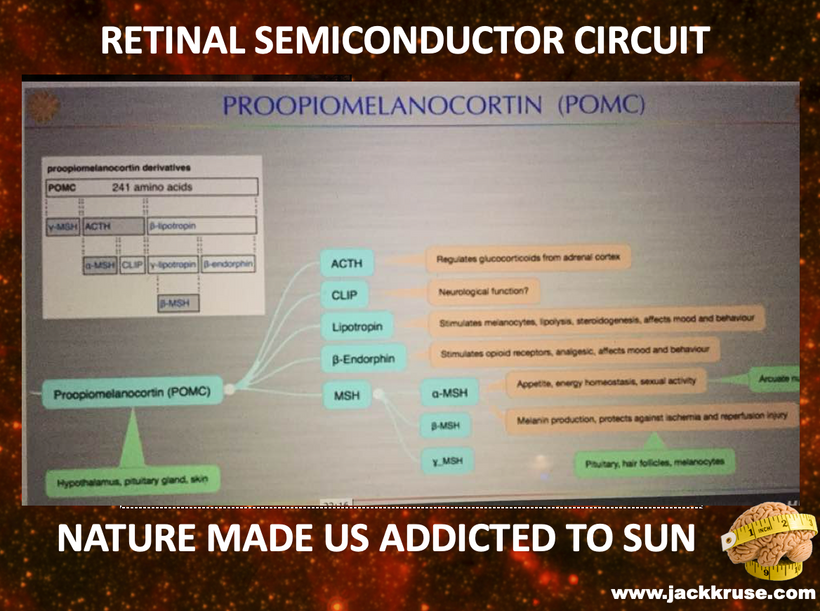
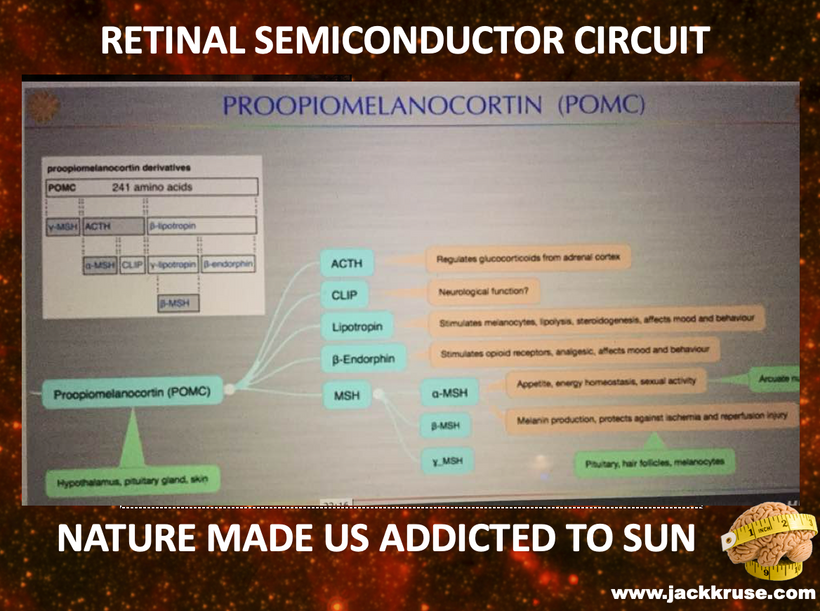
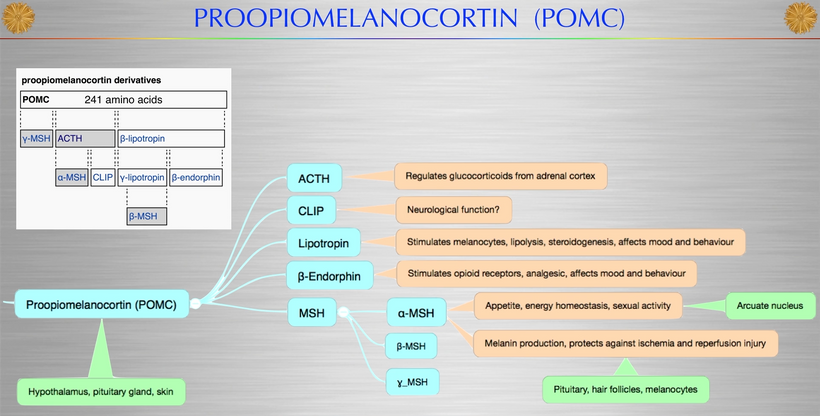
Proopiomelanocortin (POMC), as the key precursor protein linked to UV light, produces many biologically active peptides via a series of enzymatic steps in a tissue-specific manner, yielding the melanocyte-stimulating hormones (MSHs), corticotrophin (ACTH) and β-endorphin. The MSHs and ACTH bind to the extracellular G-protein coupled melanocortin receptors (MCRs) of which there are five subtypes in humans. The MC3R and MC4R show widespread expression in the central nervous system (CNS), whilst there is a low-level expression of MC1R and MC5R. In the CNS, cell bodies for POMC are mainly located in the arcuate nucleus of the hypothalamus and the nucleus tractus solitarius of the brainstem. Many cells linked to neural crest migration still contain POMC in them. Both of these areas have well-defined functions relating to appetite and food intake. Mouse knockouts (ko) for POMC, MCT 4, and MCR 3 all show an obese phenotype, as do humans expressing mutations of POMC and MCR 4. Recently, human subjects with specific mutations in β-MSH have been found to be obese too.
The MC2R gene provides instructions for making a protein called adrenocorticotropic hormone (ACTH) receptor. This protein is found primarily in the adrenal glands, which are hormone-producing glands located on top of each kidney. The ACTH receptor is embedded in the membrane of cells where it attaches (binds) to ACTH. It is also loaded with melanin and POMC.
The ACTH receptor, or MC2-R, is expressed almost exclusively in the cortex of the adrenal glands, where it regulates the synthesis and release of glucocorticoids in response to the release of ACTH by the pituitary gland. POMC expression is regulated by the end products of photosynthesis, oxygen, and glucose.
Oxygen increases POMC expression and blood glucose shuts its expression down. This is why blue light toxic people rarely tan. They cannot make melanin and their skin become atrophic. This is essentially what vitiligo is. This is facilitated by both glucocorticoids as a feedback control arm from photosynthesis (via CRH in the pituitary). Photosynthesis is mostly run via the blue and red light of the sun, not UV light. In fact, chlorophyll spectra tell us this and will make sense later.
The CNS POMC system has other functions, including regulation of sexual behavior, lactation (why many modern females can’t create breast milk colostrum), the reproductive cycle (infertility), and possibly central cardiovascular control (arrhythmias). This is why so many humans now have a-fib for no reason. They lack sun.
WHAT IS A G-protein coupled receptor?
G-protein-coupled receptors (GPCRs) are integral membrane proteins that are used by cells to convert extracellular signals from the environment into intracellular responses, including responses to hormones, and neurotransmitters, as well as responses to vision, olfaction, and taste signals. The human melanin system uses them as well.
The GPCR receptors are implicated in numerous important physiological phenomena from the regulation of inflammation to the binding of neurotransmitters. The binding of neurotransmitters is currently emerging as a new application of vibration-assisted electron tunneling in biophysics research. Electron tunneling in these contexts has been investigated as an alternative to the lock-and-key mechanism, a shape-based explanation of receptor binding.
There are 4 types of GPCR’s
GPCRs in vertebrates are commonly divided into five families on the basis of their sequence and structural similarity:
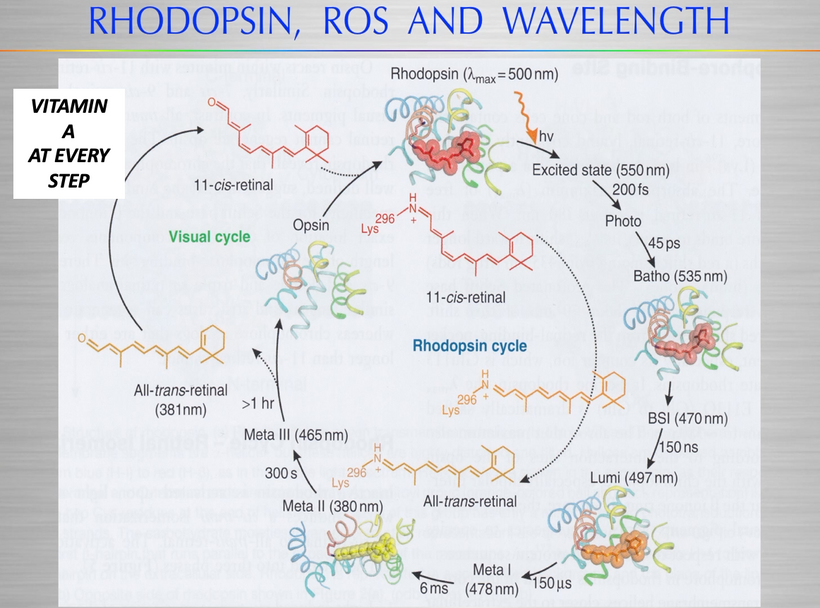
1. Rhodopsin (family A uses retinal)
2. secretin (family B)
3. glutamate (family C)
4. adhesion
5. Frizzled/Taste2
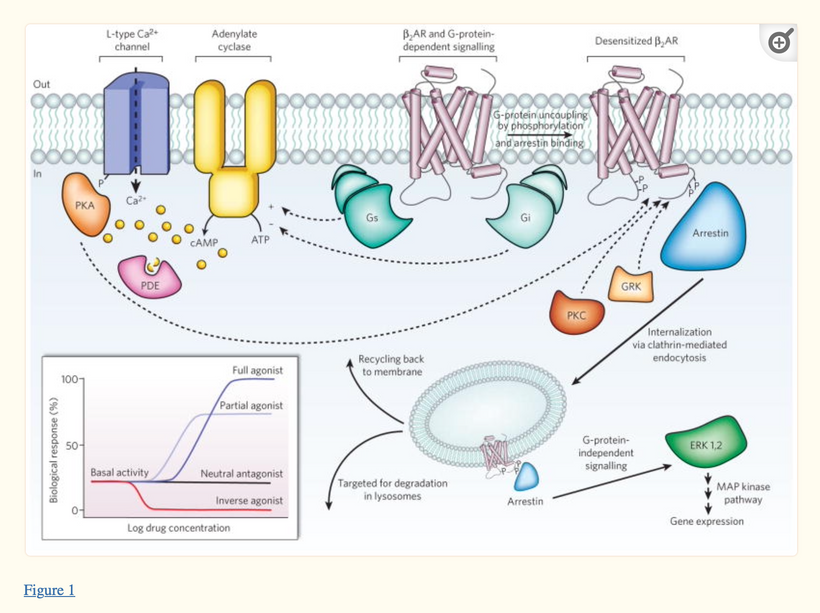
Modern animal models and pharmacological studies have shown the importance of the MCH system as a potential target in the treatment of appetite disorders and obesity as well as anxiety and psychiatric diseases. Two G-protein-coupled receptors (GPCRs) binding MCH have been characterized so far in humans
The first, named MCH-R1 and also called SLC1, was identified through reverse pharmacology strategies by several groups as a cognate receptor of MCH. This receptor is expressed at high levels in many brain areas of rodents and “primates“ and is also expressed in peripheral organs, albeit at a different rate.
Mammals with melanin damage who live under blue light cannot clear photo-damaged proteins from cells because they cannot make UV light properly.
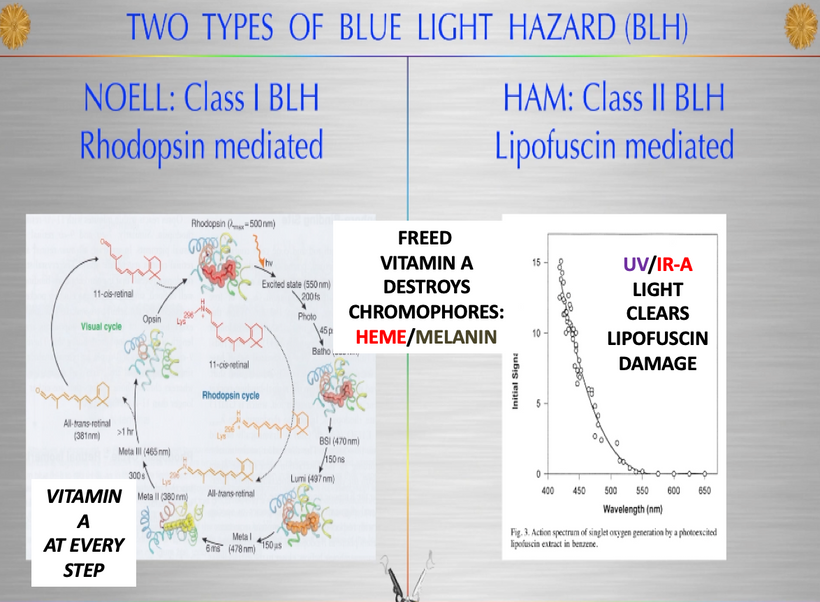
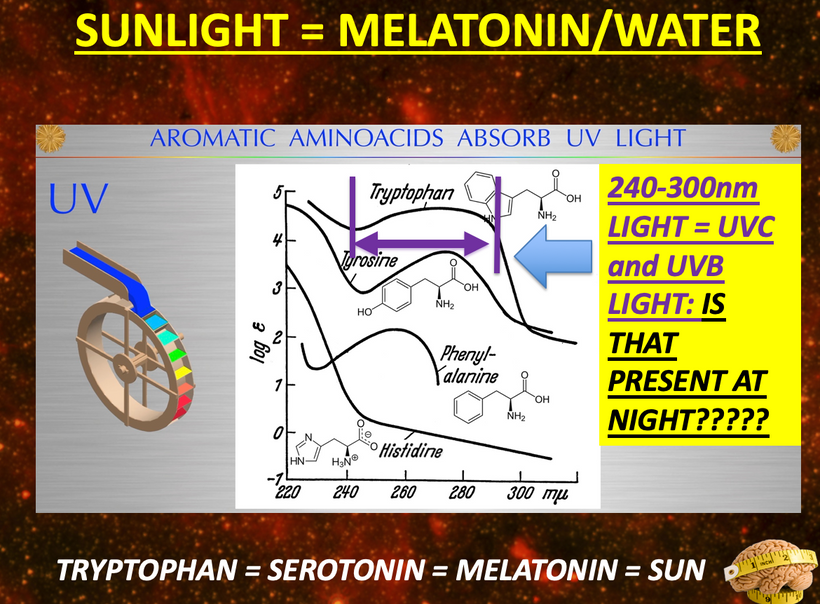
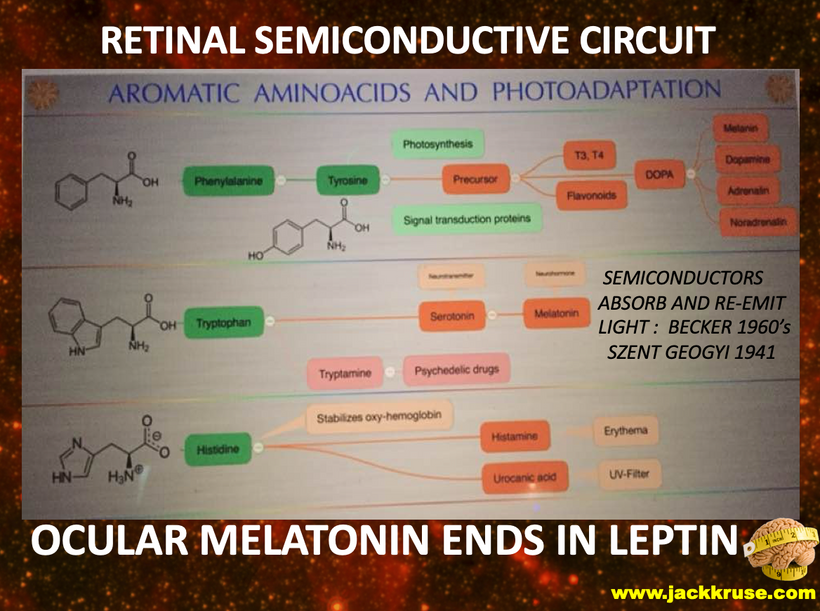
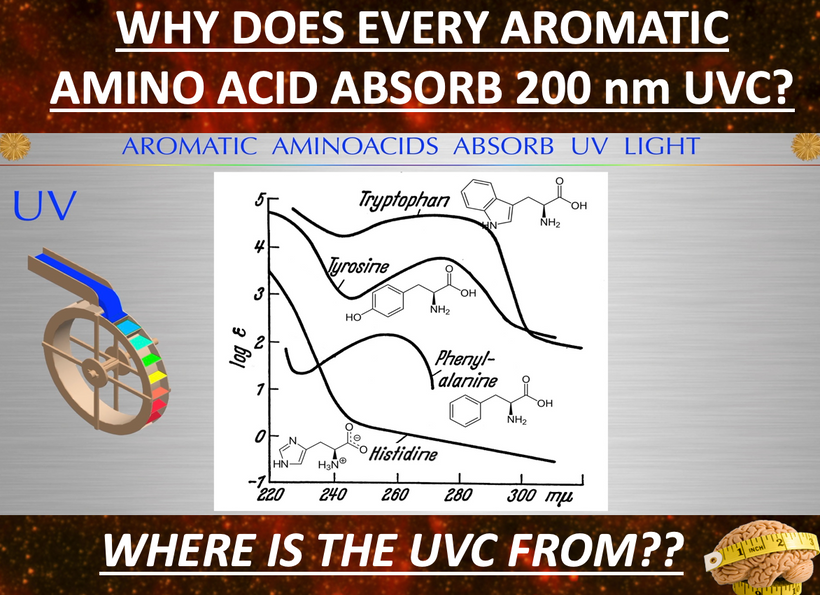
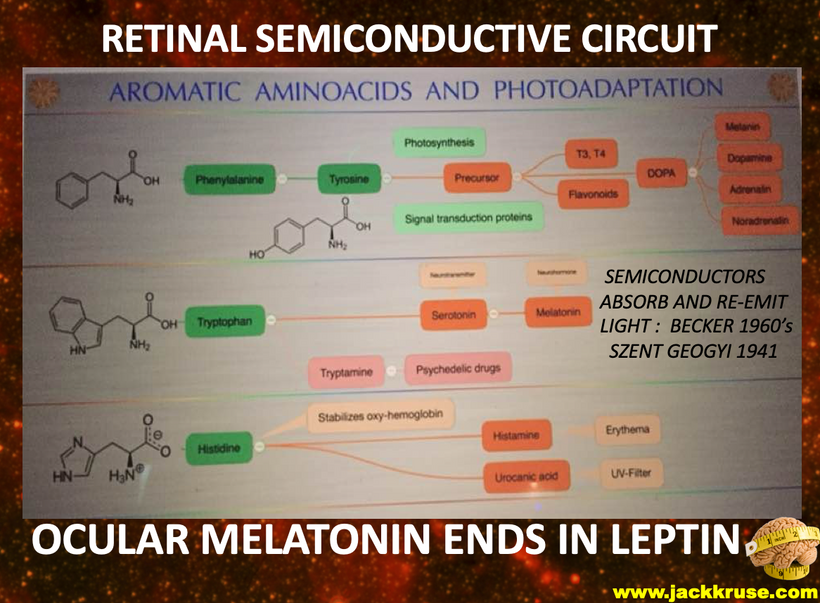
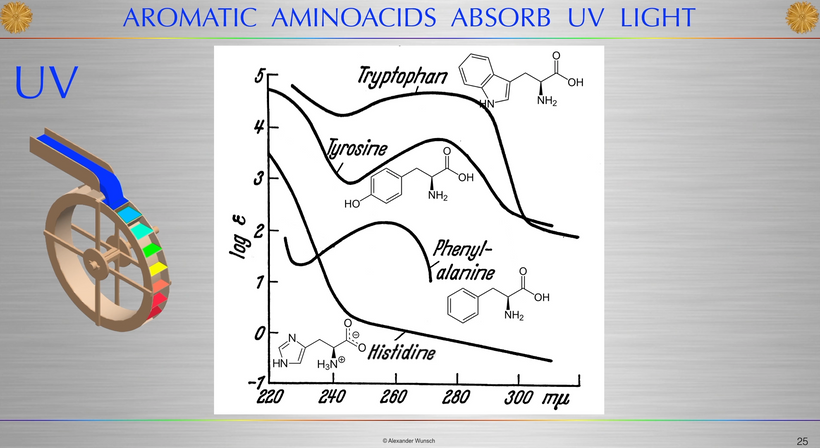
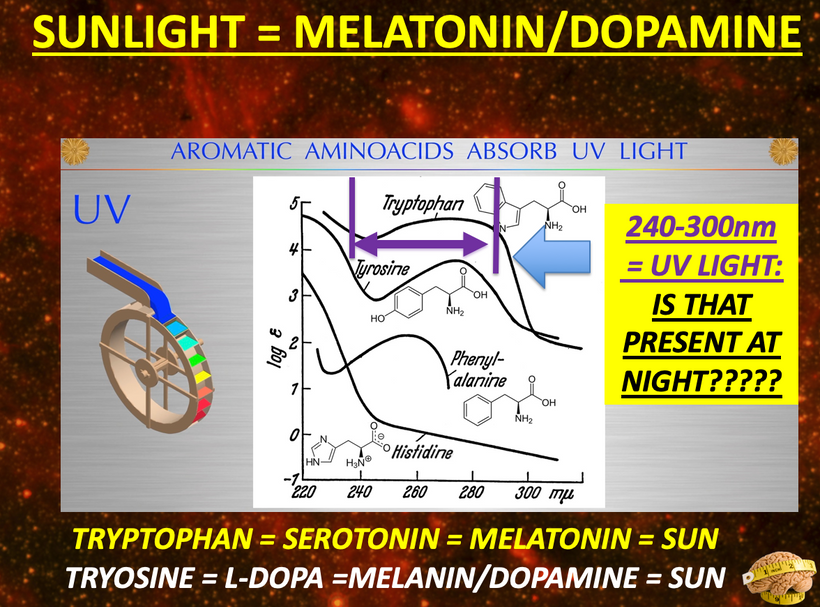
The rate is linked to the metabolic rate and redox potential of that tissue. High redox states create optimized creation of VUV-IR-A light at melanin surfaces. VUV light goes down to 150 nm light. This explained to me why all aromatic amino acids selected by evolution and coded for in DNA are used at critical points in cellular circuits that control biochemistry. They offer OPTICAL control of metabolism. This links directly back to POMC and alpha MSH operations at surfaces in our body plan.
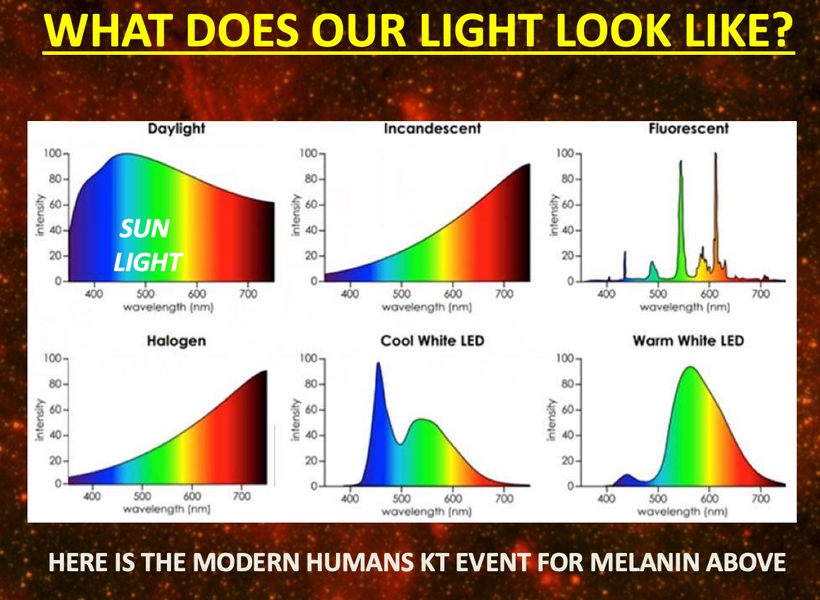
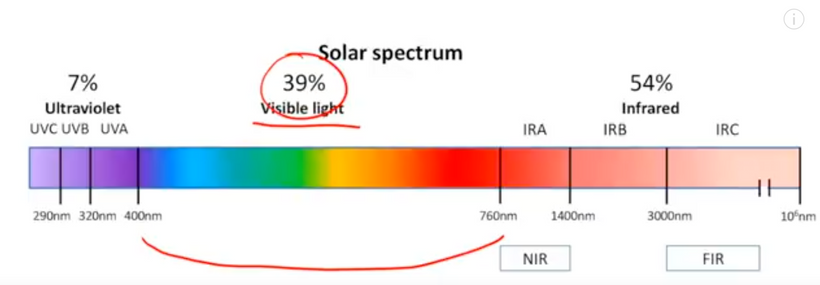
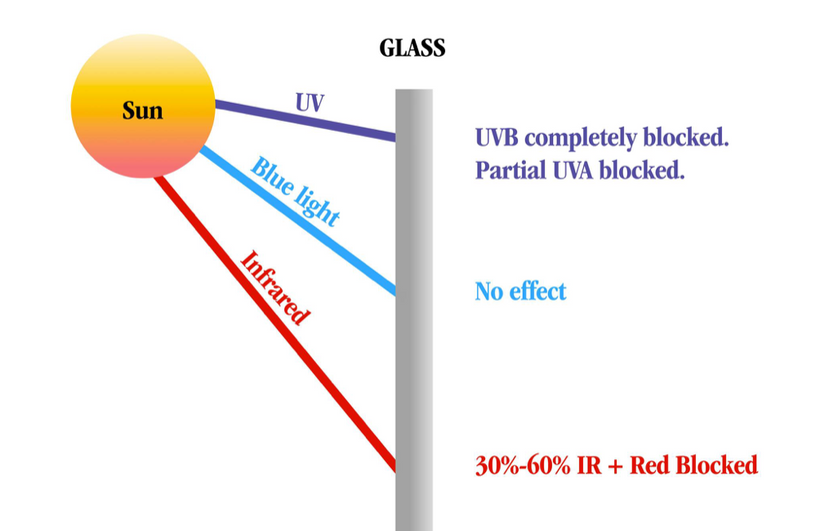
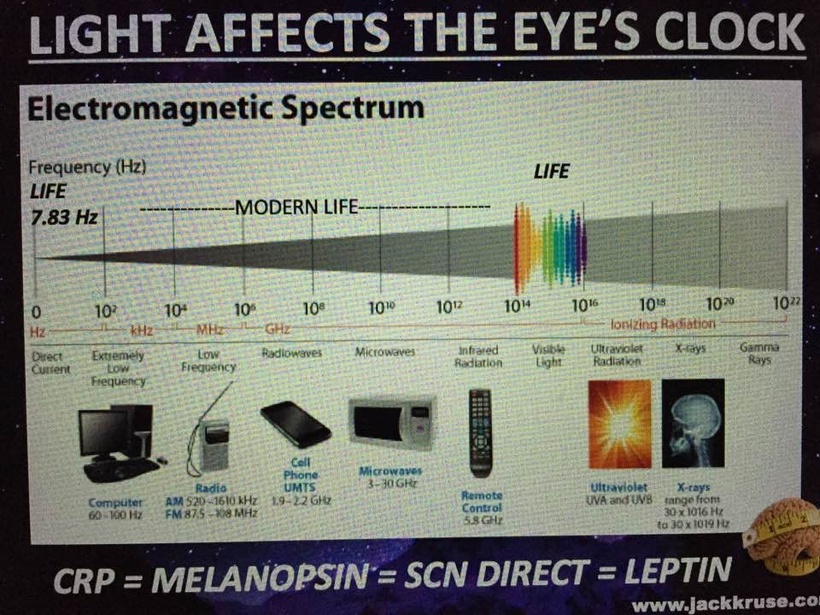
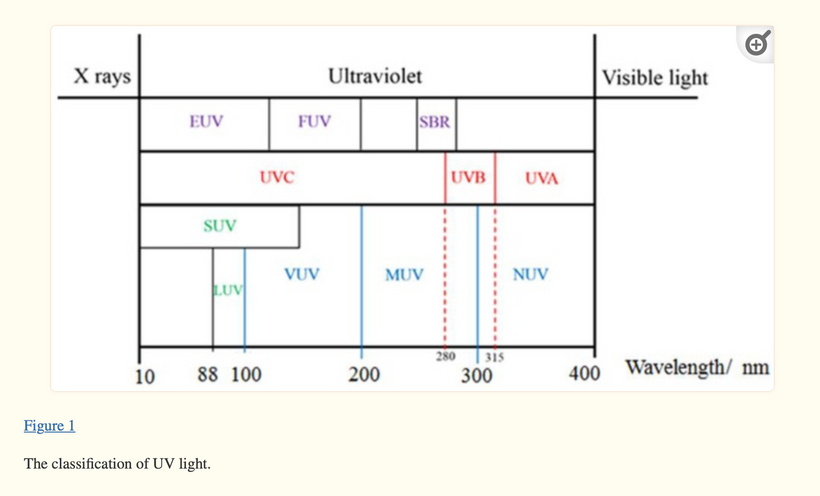
UV light is a general term for a specific band of electromagnetic radiation with a wavelength range of 100–400 nm and an energy distribution range (band gap) of 3.1–12.4 eV. Figure 1below shows the common classification of UV light, which can be divided into the following bands:
1. UVA (400–315 nm),
2. UVB (315–280 nm),
3. UVC (280–200 nm)
4. VUV (200–10 nm) = cells seem to make this type of light & terrestrial sun doesn’t
When solar UV radiation passes through the ozone layer, the 240–280 nm wavelengths are strongly absorbed. Hence, such radiation is almost non-existent in the atmosphere near the ground and comprises the so-called solar-blind region (SBR). In the SBR, it is easy to detect a target signal, false alarms are rarely produced, and there is little background interference. It appears this is why evolution favored VUV for optical signaling of biochemistry in a cell via biophoton creation. For this reason, UV detection has an advantage over infrared and laser detection technology in cells.
UV radiation in the wavelength range of 300–400 nm has a high penetration rate and can reach the ground, so this radiation band is called the UV window to the atmosphere.
A second receptor, designated MCH-R2, exhibited 38% identity to MCH-R1 and was identified by sequence analysis of the human genome. Interestingly, although MCH-R2 orthologues were also found in fishes, dogs, ferrets, and non-human primates, this MCH receptor gene appeared either lacking or non-functional in rodents and lagomorphs.
All classes of melanin receptors are class I GPCRs (rhodopsin), whose main roles are to mediate the actions of peptides and neurotransmitters in the central nervous system. This receptor system uses Vitamin A at every step. Retinal/retinal binding proteins also have 328nm absorption spectra. UV light seems to control the Vitamin A cycle in mammals.

All neurotransmitters in the CNS seem to use quantum electron tunneling to operate based on the 2023 standards in biophysics. Remember G protein-coupled receptors (GPCRs) are integral membrane proteins that are used by human cells to convert extracellular signals (environment) into intracellular responses, including responses to hormones, and neurotransmitters, as well as responses to vision, olfaction, hearing, and taste signals. We use light to see, smell, taste, hear, and touch. Proof? Olfaction uses melanin in its system of action too.
CANCER ISN’T A REAL DISEASE, IT IS AN OPTICAL EFFECT WHERE ENDOGENOUS UV LIGHT SIGNALING IS LOST
If you go back and re-read my early Cold Thermogenesis series you will see I told you this ten years ago. Few listened. The war on cancer began in 1971 with Nixon and Ochsner. It really began when man began blocking UV light from their bodies.
My work provides a solution. I will cover that soon enough in this series.
However, examples of the action of MCH on neuronal and non-neuronal cells are emerging that illustrate novel MCH functions. In particular, the functionality of endogenously expressed MCH-R1 has been explored in human neuroblastoma (cancer) cells, SK-N-SH, and SH-SY5Y cells, and in non-neuronal cell types such as the ependymocytes. My patients with neuroblastoma and retinoblastoma get told different things from me than their centralized MDs do. The egg they came from did not have a lot of VUV-IR-A light. The oocyte’s wide-band semiconductors were defective. Did you know the leptin-melanocortin pathway controls oocyte selection (fecundity) in ALL mammals? Yes, it explains modern infertility.
What else is critical here? Cells that form the ependymal lining of the mammalian ventricular system in the CNS arise from the ciliated epithelial lining (primitive neuroepithelium)of the neural tube. The cells line the inside of your brain, yet they are loaded with POMc. The optic vesicle, an evagination of the neural tube, is initially lined by ciliated neuroepithelium. In the outer wall of the optic cup, however, the cells which elsewhere are differentiating into ependymal cells, lose their cilia and eventually produce melanin. In childhood retinoblastoma and neuroblastoma, there is the migration of cells from neural dermatomes in utero that have lost VUV creations and this stopped mitosis and allowed them to migrate to the eye or medulla and when they found other cells with VUV release they began to divide. Yes, this is how all childhood cancers form. Defective neuroectoderm due to circadian disruption in mom or dad is an issue. There is a solution for those cells too.
This migratory issue is also linked to the development of ALS and MS, in my opinion.
Indeed, modern centralized science has identified the mitogen-activated protein kinase (MAPK)-dependent that use calcium-dependent signaling cascades that ultimately contributed to neurite outgrowth in neuroblastoma cells or to modulation of ciliary beating in ependymal cells. Calcium is one of the high band gap atoms I mentioned to Dr. Huberman during the podcast. Note how the sun offers quantized control of calcium flows in the cell. This offers optical control of the VUV-IR-A spectra emitted from cells.
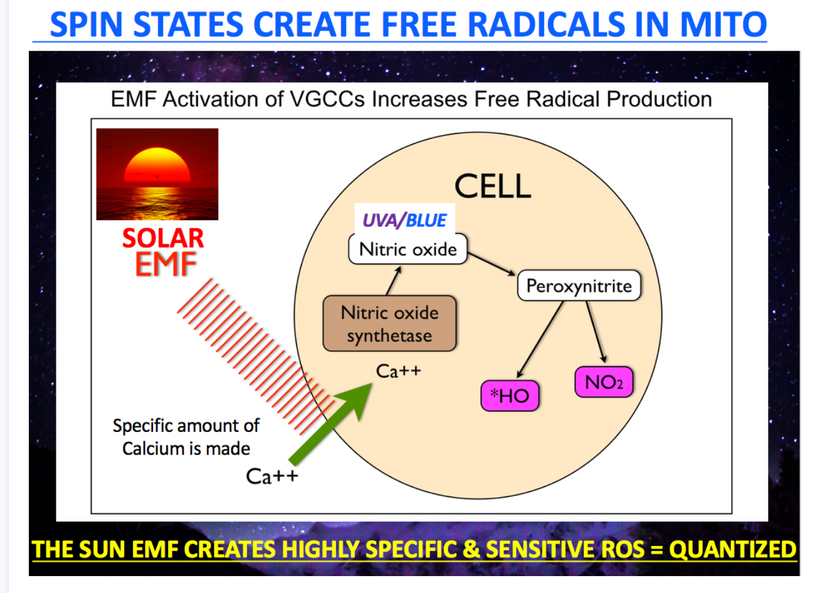
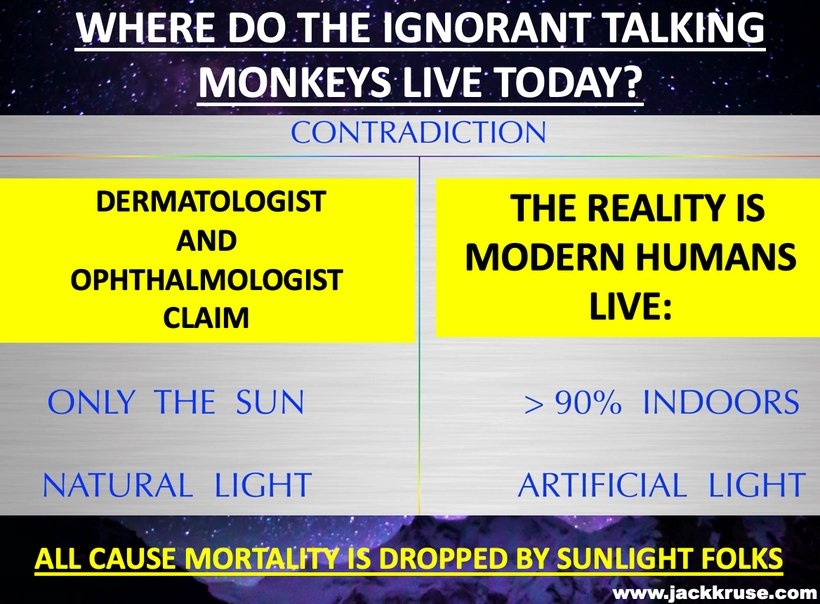
People continually repeat the lies of dermatology and ophthalmology by saying UV sunlight is dangerous but UVC sunlight is beyond toxic. Is it? Do you know anything about the Auger effect? All mammals use the Auger effect to recycle their mitochondria. Did you know that? Look at this below. Everyone seems to know that terrestrial sunlight does not have UVC light in it, yet UVC light inside of us seems to be a key way for us to recycle our failing engines. This implies that light created by wide-band semiconductors plays a big role in the energy efficiency of organs that are failing. This is exactly the opposite of the narrative of centralized medicine. What experts do you believe now? The ones who told you staying inside out of the sun and away from other humans was wise or a neurosurgeon who told you to embrace Nature and let cells do what they are designed to do?

HYPOTHYROIDISM WITH A LACK OF INTERNAL MELANIN IS A GREAT WAY TO DEVELOP AN EPITHELIAL CANCER OR GET A NEURODEGENERATIVE DISEASE.
WHAT?
Blue light exposure is the perfect way to get this. See the blue highlight below.

The fastest way to get epithelial cancer with artificial light at night also has hypothyroidism induced by blue light toxicity in the eye via the central retinal pathways. This raises your TSH and causes hypothyroidism. If you have glaucoma or myopia you are at risk too. This is why people with hypothyroidism have more aggressive melanomas and Parkinson’s disease than those with normal thyroid function.

It is also why melanoma in those with hypothyroidism show up on skin that is not exposed to the sun. I wonder when people will realize why this really happens. POMC creation is lacking in the eye and skin of these people. I shared this with Rick Rubin and Dr. Andrew Huberman. Both of them were stunned.
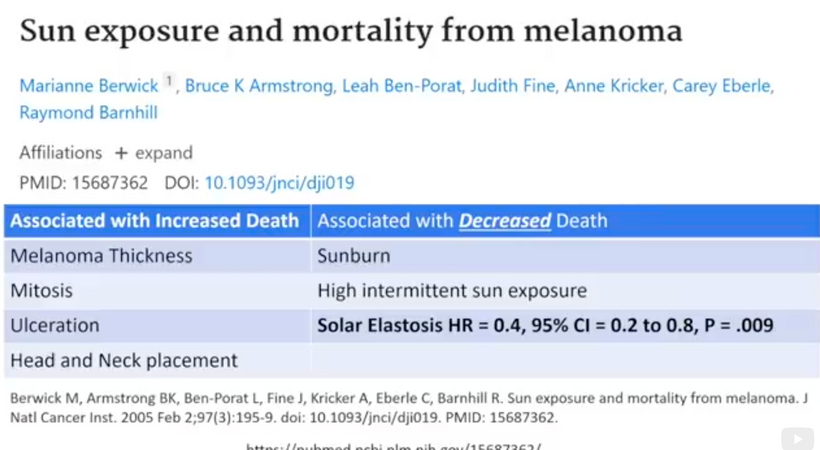
Have you figured out why now good thyroid function predicts a better melanoma prognosis yet? The literature shows a very interesting connection between TSH and melanoma. A low TSH (around 2.0) is correlated with a slower progression of melanoma. People with hypothyroidism carry a much higher risk of melanoma risk because of how POMC cleavage occurs. Why? Look at the slide ABOVE CAREFULLY.
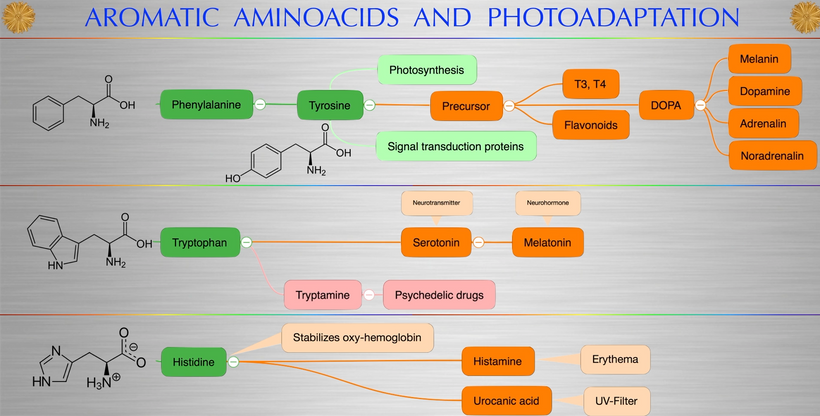
What creates T3 and T4? Tyrosine and UV light. Do you see that phenylalanine & tyrosine, both aromatic amino acids, can also be used as substrates via DOPA? DOPA can be used to renovate melanin inside your skull if VUV light is present in that tissue. (note the absorption spectrum of these amino acids) The sun is not toxic. The advice you have been given for 100 years is.
Conversely, when your hair/skin/integument loses its melanin and you’re hypothyroid it will cause dopaminergic cell loss in your skull because these cells will undergo de-differentiation to their embryological forms. (key point) Those cells in the substantial nigra cannot migrate out of the midbrain if UV light is present in your environment via your skin and eyes. (also explains why Parkinson’s begins in the eye). The irony is that the connection is published but no one seems to understand why. Now you do for 5 bucks a month = decentralized medicine 101

If T3 and T4 are optimized it means that the cells inside of you are still making some level of VUV-IR-A from their wide-band semiconductors in your eyes. If you have skin melanoma at the same time it means that the area of neuroectoderm with cancer tracks back to a neural dermatome where melanin is being destroyed inside of you but your prognosis is much better because your eyes have created enough T3 and T4 to make VUV-IR-A to stop melanosome migration.
POMC is made in your eyes and close to your RPE. POMC gives rise to several biologically active peptides that are expressed primarily in the pituitary and brain. These peptides aren’t limited to those tissues and this explains why high TSH hypothyroidism melanoma cases are more dangerous from a prognostic perspective.
Sunlight in the visible spectra stimulates POMC with the help of the TSH. So if your eyes are in the light but your thoracic skin isn’t, that is where your melanoma will show up. Conversely, if you have vitiligo and you don’t have hypothyroidism it will be harder to re-pigment your skin. People who live under fake light do not make POMC or TSH well and this means mitosis is defective in their cells. When mitosis is defective in mammals their cells do not divide and they become mobile and invade other tissues because they are looking for a UV light source. We call this metastasis. Mammals invented metastasis to survive the KT event. That is the story of melanoma I laid out to Rick and Huberman. They were stunned. Look at the extreme top right of the slide below. That is a story of you going from chimp to human a lot quicker than you can imagine. Once primates got enough melanin inside of them they used the power of the sun and three tectonic plates with a lot of DHA from the marine chain to build their frontal lobe circuits. We used melanin to do it. Every last neural tract in those lobes is controlled by dopamine and noradrenaline. All were made from melanin biology.
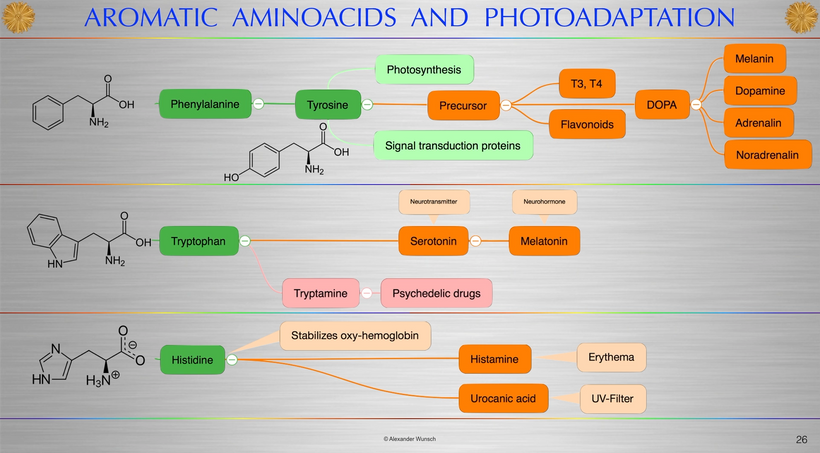
THE ENTIRETY OF HUMAN POMC EVOLUTION CAN BE VISUALIZED BELOW
Open it and watch it before going on.
https://twitter.com/intothefab/status/1633890797107007494
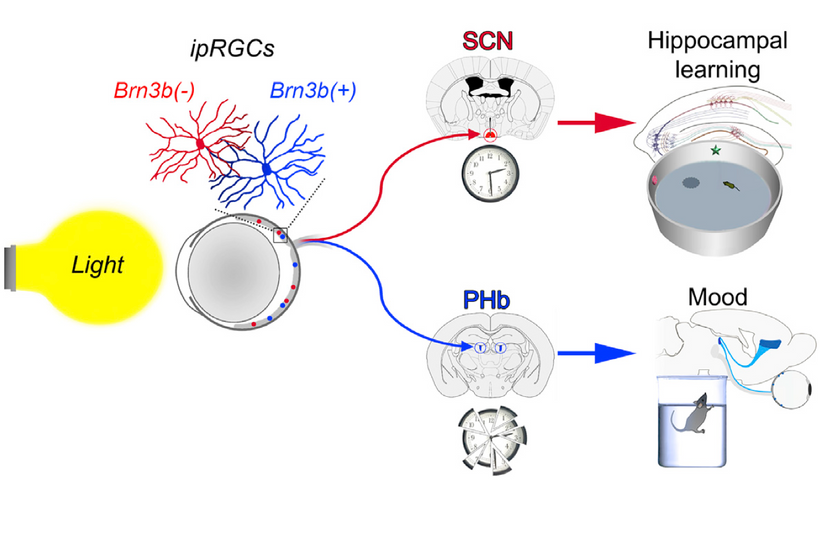
Dr. Huberman is very interested in mental disease and the use of psychedelic drugs. I explained to him why those drugs work for mental disorders. The drugs provide a tryptamine substrate that normally can only be made in cells after VUV-IR-A light emission creates them from tryptophan (see above slide). So psychedelic drugs replace missing UV light from the sun. Most people with mental diseases are solar deficient at some level. The effect is almost always linked via their eyes, contacts, sunglasses, glasses, and life built around nighttime light. (see below)
What else did I mention to Dr. Huberman and Rubin about the periodic table? I told them all of life’s wide band-gapped semiconductor components would be found in a specific area of the periodic table because they all make VUV-IR-A light inside of us. Phosphorous is one of those atoms.
The most striking example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, while the emitted light from the cell is in the visible region, which gives the fluorescent substance a distinct color that can be seen only when exposed to UV light. Cephalopods and scorpions do this and so do chameleons. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Do mammals and birds exhibit this? Yes. Mammal skin and hair emit UV light and birds do too. All the pieces fit.

Cooling mammalian tissues makes them fluoresce more. This is obvious if you are a hunter in winter and use a UV as a tracker. Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. In cases where the semiconductor is a wide band semiconductor if it is doped with a group 2-4 atoms in them, you can get band gaps that cover even the VUV- light to 150nm. This is what mammals do. It explains why they made it through the last extinction event. The same thing is true with feathered dinosaurs. Melanin was the key. This is why the leptin-melanocortin pathway is in every mammal on Earth. Early mammals 210 million to 65 million yrs ago had the majority of their melanin on their exterior surfaces. Modern complex mammals brought it to their insides as well. This is why you have heard me say over and over again that before I am done with this thesis it will become obvious that what happens on our surfaces is far more important than what happens inside biochemical pathways.
MAPK PATHWAYS EXPLAIN THE BIRD PICTURE ABOVE
Birefringence is a non-linear optical effect seen in the bird above. Cephalopods have it too. It is tied to water’s ability to change its polarization by light release inside our bodies.
Mitogen-activated protein kinases are protein kinases that phosphorylate their own dual serine and threonine residues (autophosphorylation). This means in response to endogenous light cells create MAPK dopes our internal semiconductors with phosphorus to change the ability and phenotype of the tissue in question. Wide-band atoms were and are the key to Darwin’s natural selection. It is not genes. Genes only provide semiconductive proteins.The atoms next to those gene products are what control life and give the band gap of the semiconductor in that tissue. That band gap correlates with the light spectra it releases to water, and this creates the biophoton signals that create the color and frequencies of light that control cellular processes optically. That is what you are seeing in the bird above and what Dr. Huberman sees in his cephalopods in his lab every day. He did not know how they did it and now he does.

Research has confirmed and found on their substrates, to activate or de-activate their target (Johnson and Lapadat, 2002; Peti and Page, 2013). Accordingly, MAPKs regulate important cellular processes such as proliferation, stress responses, apoptosis, and immune defenses in mammals. (Dong et al., 2002; Liu et al., 2007; Arthur and Ley, 2013). MAPKs are ubiquitously expressed and evolutionarily conserved in eukaryotes (Kyriakis and Avruch, 2001; Kyriakis and Avruch, 2012; Peti and Page, 2013). The activation of a MAPK cascade occurs in a module of consecutive phosphorylation!!!
The conventional ideas in biochemistry tell us that the hydrolysis of the high-energy phosphate bond produces only -7.3 kcal/mol. I explained this to Dr. Huberman and told him this number breaks the second law of thermodynamics by 500 fold. Shout out to Gilbert Ling. The hydrolysis of ATP does not explain where a cell gets its energy. When you compare this value to the almost -60 kcal/mol of oxidized NADH, it becomes clear the power found in a cell is electronically induced by solid-state physics and not driven by the hydrolysis of ATP.
I told Dr. Huberman that the collateral information of the ideas I shared with him would stun him when he followed the breadcrumbs from our podcast. Here is the monster blog after I let the cat out of the bag to him. You will know then he will because his head is still spinning. I was so provocative that he wants to come to visit me in my clinic to go deep into some of these things in this blog. The two hugs he gave me after our recording tells me I landed my punches.
IT IS ALWAYS ABOUT YOUR LIGHT CHOICES FOLKS
A lack of UV light from our interior cells that make it causes cells to GAIN MOBILITY. Gaining mobility in a cell line is a synonym for neuroplasticity.
Neuroplasticity is built into every neuroectodermal tissue in mammals.
POMC drives this program in the adult form of mammals. SUNLIGHT CREATES MORE POMC = SUNLIGHT CREATES MELANIN and a whole lot more. This is why my Leptin Rx affects so many other modern diseases.
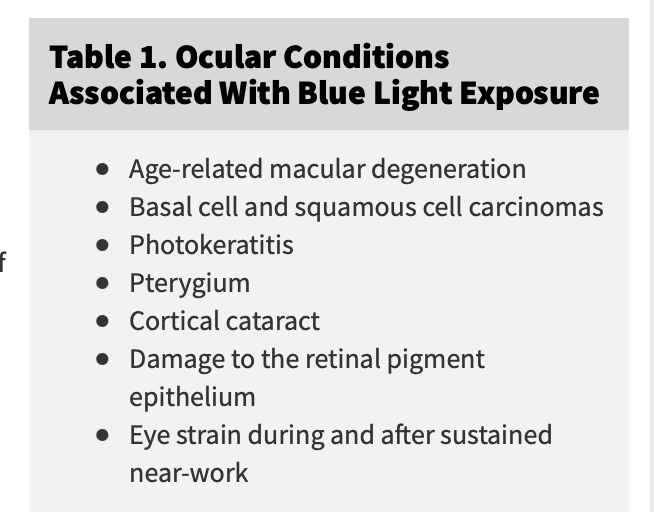
Disordered optical signaling is behind every form of arthritis on planet Earth. Sounds crazy until you get the perspectives built into this blog. Just look at the paper below and the blue highlighted areas. (Shout out to my buddy Dickran)

Everyone with joint diseases needs a melanin renovation program. This explains why older people need more sunlight not less and why arthritis pre-tech era was always an elderly disease. Now we can see young people with massive levels of arthritic changes when they bury the sun and embrace technology’s blue light and RF and microwave emissions. Blue light has to be protected by IR-A light to renovate all heme and melanin semiconductive proteins. Adding 460-500nm light to improve cognitive function does not work without IR-A light. (Dave Asprey fail) The blue light that energizes the neocortex is never present on Earth without IR-A light and that is lost on poor thinkers. (below)
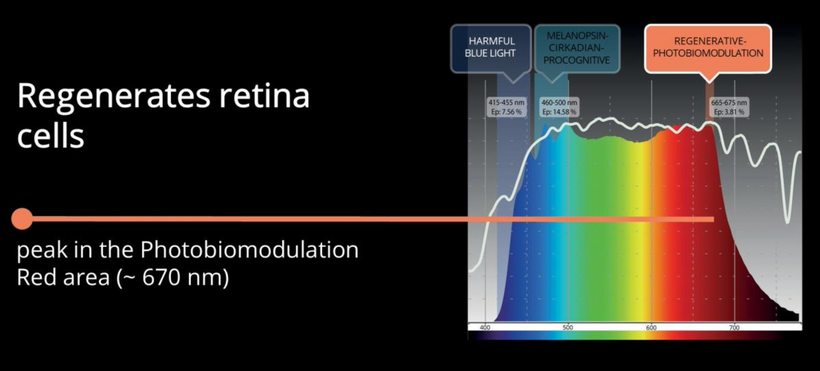
NOTICE WHERE POMC IS IN YOUR SYSTEM
POMC is expressed in several components of the skin including melanocytes, keratinocytes, heart, adipocytes, neurons, immune cells, and dermal microvascular endothelial cells. (below).
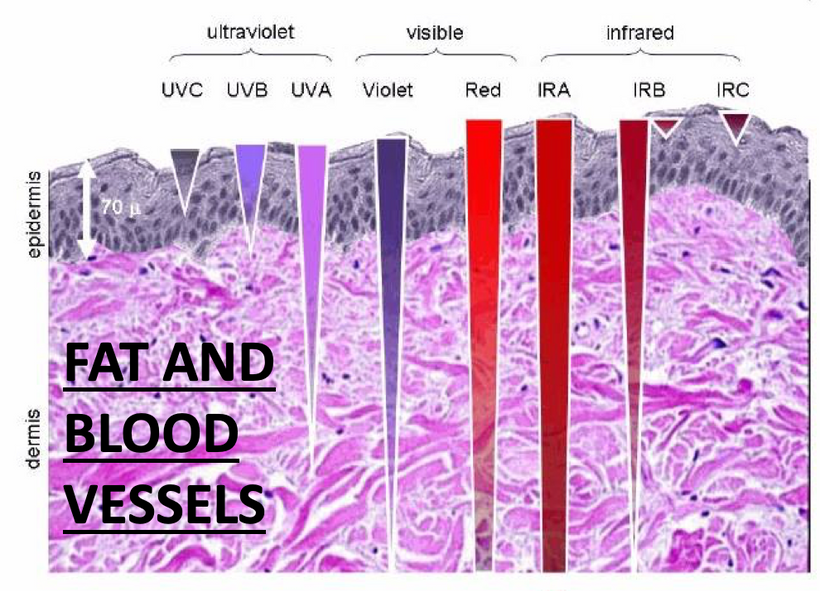
Just look where POMC is in most of our tissues!!! The skin on your abdomen has huge implications for all gut dysbiosis diseases. Your functional medicine docs are as full of shit as are your allopathic providers are on sunscreen and statins. They are not packing your parachute at all. They are packing their wallets.

WHY DOES POMC HAVE SO MANY CLEAVAGE PRODUCTS?
Nature does not make errors and she embraces paradox to ensure survival under the sun on earth.
Why are ACTH and POMC linked as cleavage products? Glucose is created by photosynthesis. In fact, all food webs link back to the sunlight. Photosynthesis does not use UV light to create food. It uses everything but UV light to do it.
One interesting thing I brought up on day two to Dr. Huberman does he ever find it unusual that the first step in photosynthesis is to charge separate water. I asked him did he know what the energy density required was to do this. He did not. I told him that it is 12.06 eV. That means water needs soft X-rays to make food. We all know that is not happening, so it raises the question of how does nature do it. I asked him in the podcast at this point does Nature make any mistakes. Why doesn’t photosynthesis need soft X-rays to split water as step one in photosynthesis?
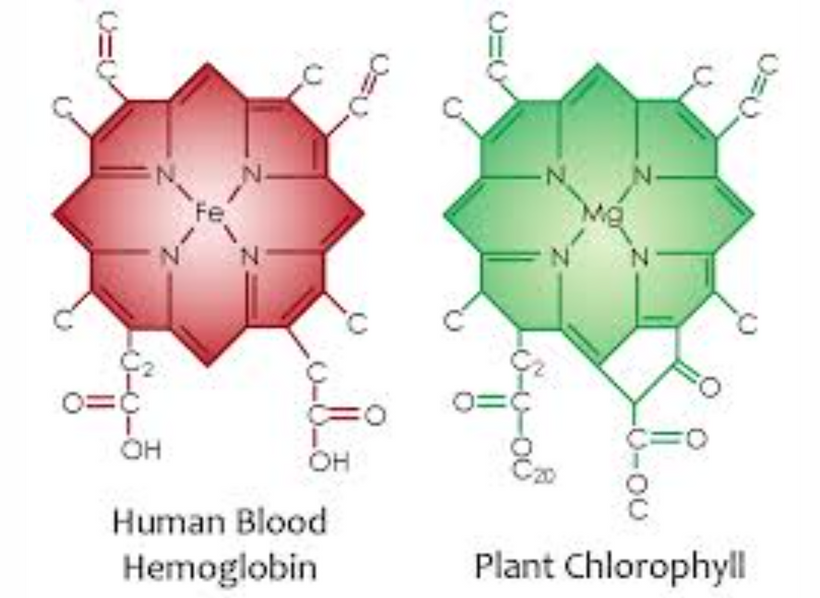
Cells used magnesium inside a nitride cage to act as a wide-band semiconductor BEFORE the Cambrian explosion to create food and oxygen. This changed the conditions of existence on Earth. Now we can really understand Darwin’s thesis when we add in solid-state physics. I told Huberman that chlorophyll and hemoglobins were key semiconductors developed on Earth during the Great Oxidation event.
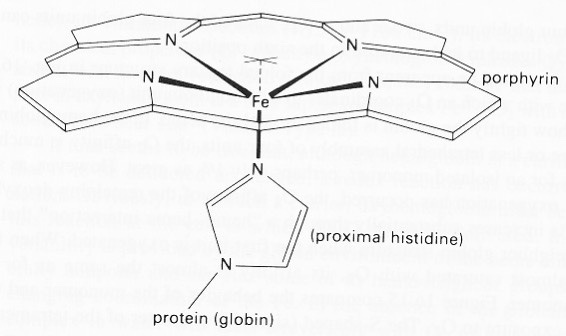
I believe leptin came after both and then melanin became the dominant player post-KT event. Melanin renovation requires an outdoor existence because of the creation of POMC on our skin and in our eyes. My melanin renovation Rx requires optimized hemoglobin dynamics to keep the melanin in our heads optimized to create VUV-IR-A light. This has to do with iron.
Since photosynthesis does not use UV light how did mammals upgrade their system? They used hemoglobin to boost their POMC system post-KT when UV light was absent. This drew melanin inside their bodies from their integument. They optimized their central melanin system to harvest more energy from the sun than the sun provides.
I told Rick and Andrew over and over again that nature does not make mistakes. Rick agreed, but Andrew did not. I told him I knew he would say this because of the centralized mindset. The proof I am right that nature does not make mistakes and there there are no coincidences is below.
KEY POMC POINT: This POMC expression is regulated chemically by both glucocorticoids as a feedback control arm from photosynthesis (via CRH), and also by ultraviolet radiation. Mammals used the return of UV light post-KT to rebuild their circuits just as Intel uses photolithography to make new circuit boards on silicon wafers.
Read that again. UV light controls the amount of POMC created on our surfaces.
The more UV light we get on our skin, the more melanin inside our skulls can innovate new solutions the environment would throw at us. The more UV light we get the more POMC we get = the key to the melanin renovation Rx my farm members get from me.

The adrenal gland makes glucocorticoids. Glucocorticoid hormones regulate essential body functions in mammals, and control cell metabolism, growth, differentiation, and apoptosis. Natural endogenous glucocorticoids are produced by the cortex of the adrenal gland. The adrenal glands are organs located immediately above our kidneys. The adult adrenal medulla is loaded with melanin in humans, in case you did not know this.

Understanding neurulation which is the migration of neurons during embryogenesis tells us a lot about the migration of neurons when adult tissues lose their ability to generate UV to create mitosis. Adrenal fatigue is like a disease that causes cell loss in the adrenal medulla. The dorsal aorta, the first major blood vessel established during embryogenesis, expresses BMP signals to direct migration and lineage segregation of neural crest cells to form into the adrenal medullaand the sympathetic ganglia. Schwann cells, neural crest–derived glia that ensheathes peripheral nervous system neurons, direct patterning of arterial vasculature parallel to sensory nerves through the expression of CXCL12 (also known as SDF1). Neural crest cells retain a relatively broad developmental potential as they begin migration and their ultimate fate is strongly influenced by local factors. Guess what the local factor is? Light frequencies cells make that control another non-linear process called ferroelectricity. More on that in the series later. That is the key to making sense of Becker’s expoeriments.
Glucocorticoids downregulate POMC expression and this is coupled with hair follicle cycling in ALL mammals. This is why hair loss and sweating were key findings in the Leptin Rx. This gave me a big clue that when photosynthesis was interrupted and it was cold, mammals would have to create blood glucose in their cells from their fat stores during the cold spell post-KT collision to survive. It was here I learned that blood sugar and insulin cause melanin to be reabsorbed and moved. They cause melanin to move in the neuroectoderm. Instead of being recycled melanocytes undergo a form of diapedesis. This is like reverse metastasis. Mammals are experts in the metastasis of their melanin. This was how they survived a cold environment with no UV light.
Melanocytes can move toward ANY UV light stimulus just like a plant would. It is equivalent to phototaxis in plants when plants grow toward the sunlight stimulus.
The protein precursor known as proopiomelanocortin (POMC) holds within its structure numerous biologically active peptides, including adrenocorticotropic hormone (ACTH), β-lipotropin, β-endorphin, and α-MSH. These peptides are proteolytically released from POMC by the proprotein convertases. Those PC cleavages are controlled optically by the light that cells release inside of us (VUV-IR-A light).
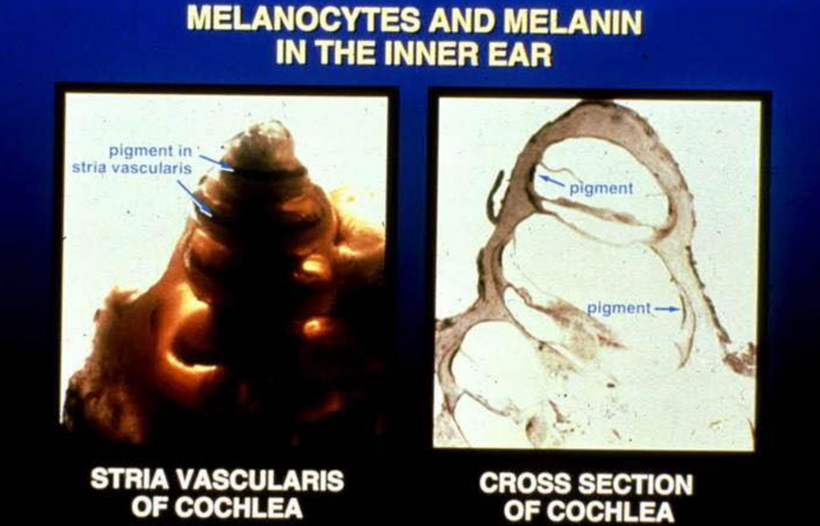
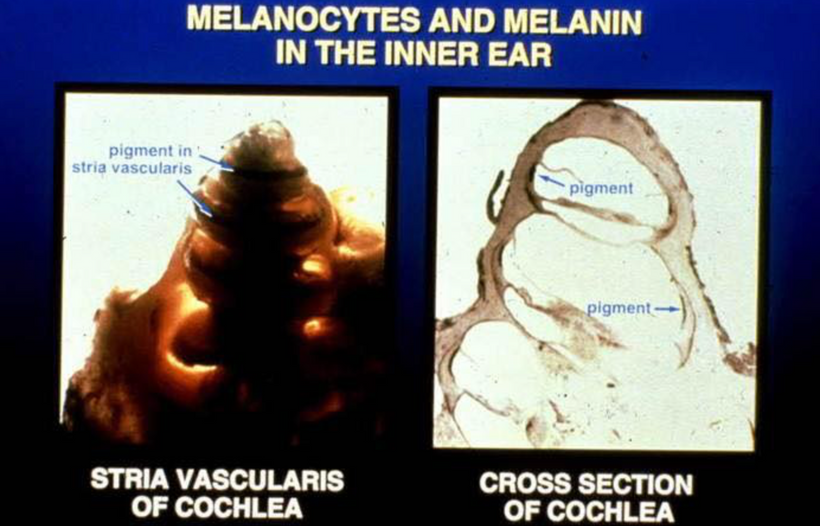
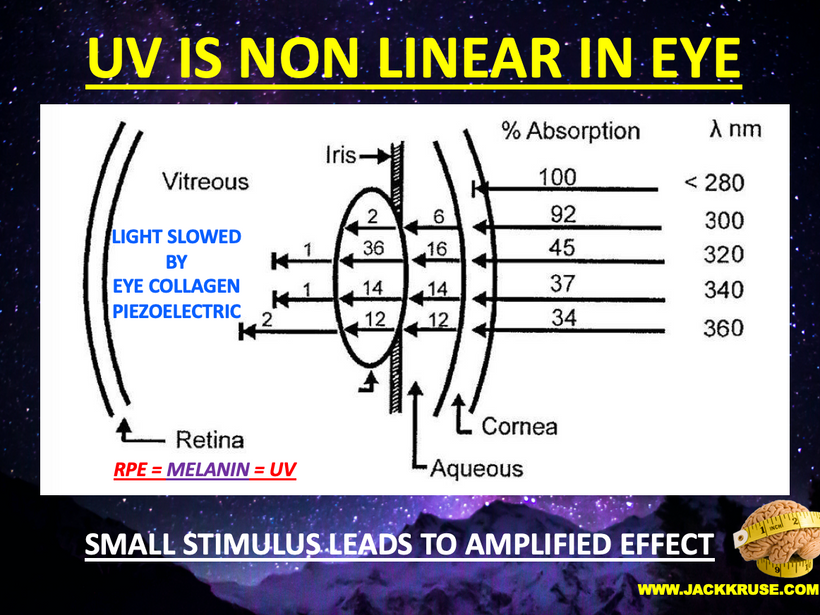
POMC is synthesized in the corticotrophs and melanotrophs of the anterior and intermediate lobes of the pituitary, respectively, as well as in peptidergic neurons in the arcuate nucleus of the hypothalamus. These are the targets of terrestrial sunlight that is FIRST focused on the RPE of the retina or melanin in the skin. In our guts, the skin above the microbiome targets enterochromaffin cells to make the magic happen there. The RPE of the retina is loaded with melanin and so is the iris. (below) The reason why the eye sclera is white (special collagen) is that it is designed to reflect all light and focus sunlight through the pupil as a perfect black box radiator. The vitreous eye collagen slows light via Fermat’s law. (Vermont 2018)
This POMC hormone helps maintain blood sugar levels, protects the body from light stress, and stops (suppresses) inflammation centrally and peripherally on a surface where POMC is found in humans. Three similar peptides called alpha-, beta-, and gamma-melanocyte stimulating hormones (α-, β-, and γ-MSH) are also cut/cleaved from the POMC protein. These peptides are the key to the MELANIN RENOVATION Rx.

Another shocking effect of this realization explains all autoimmune conditions today when you realize technology has brought humans inside and in front of screens and devices that emit blue light, RF, and nnEMF. This subtracts the sun and explains why every human autoimmune condition is associated with low Vitamin D levels. It is a proxy for this story. The action of the remainder of those cleavage products from POMC is why humans get autoimmune conditions when nnEMF is brought into their environment. All of them act on our immune system cells in novel ways. Alpha, beta, and gamma MSH have a big role in vasopressin release with light stress. I already gave you a blog in January this year on that topic but many of you did not see this coming. POMc is loaded in vasopressin neurons in the hypothalamus.

This is the base-level understanding built into my Leptin Rx. None of this was present in the original leptin blogs. If you don’t believe it, go re-read them and then read this one. I explained this to Huberman and Rubin this past weekend in the ten-hour podcast we recorded. I told them people would have never understood the complexity of this blog until I laid out all parts in front of them. Each part had to be taught piece by piece so they would reject centralized healthcare ideas for good.
Diapedesis in WBCs is a remnant of this activity in mammals. So is the ability to regenerate our fingertips. Becker was the one who showed me humans could do this because of his semiconduction papers in the 1960s I reviewed as a young neurosurgery resident.
Today metastasis also remains a remnant function in our immune cells that harms us in oncogenesis. Oncology has it all wrong. Diapedisis of WBC during infectious invasion is another example of modern mammalian metastasis. WBCs retain the ability to move because their nucleus turns off mitosis during its life span. WBC contains POMC and this gives our immune system the same ability our ancestors had at the KT event. UV light can be abolished by the divalent atoms inside the WBCs to allow the WBC to go after invaders in our tissues. When mitosis is turned off they become the soldiers to fight your battles in your immune response.
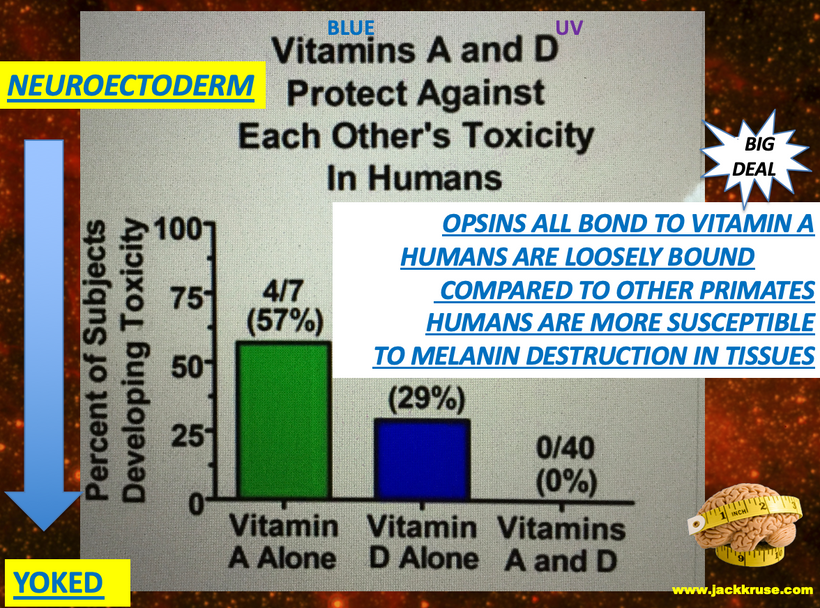
The retained neuroplasticity of the neuroectoderm made their melanosomes mobile. We see this connection in labs when we check Vitamin A to Vitamin D levels in them. Both of these are proxies for melanin damage inside their skulls, skin, or in their adrenal glands where melanin resides. This is what my patient get during workups. This is decentralized medicine 101.
This meant the change in light frequency caused melanosomes to move to the interior of their bodies, where cells deep inside of them were still making VUV-IR light from their wide band semiconductors like collagen. Huberman’s eyes widened when I explained to him that Becker proved in the literature that bone conduction was semiconductive and that collagen was the N-type semiconductor. I told him I realized that since the brain only used 20 volts it had to be based on wide-band semiconduction because of what modern lighting engineers are doing with them to save energy. Modern Solid-state lighting also uses wide-bandgap semiconductors because they provided the potential to reduce the amount of energy required to provide lighting compared to incandescent lights.
The wide bandgap semiconductor design would also bring the electronic transition energy into the range of the energy of visible light containing UV spectra. This explained why all living cells emit ultraweak-UV light to signal. This validated Fritz Popp’s findings.
Here is how Dr. Popp explained it in his own words before his death in 2018:
“We know today that man, essentially, is a being of light. And the modern science of photobiology is presently proving this in labs all over the world. In terms of healing, the implications are immense. We now know, for example, that quanta of light can initiate, or arrest, cascade-like reactions in the cells, and that genetic cellular damage can be virtually repaired, within hours, by faint beams of light. We are still on the threshold of fully understanding the complex relationship between light and life, but we can now say emphatically, that the function of our entire metabolism is dependent on light.”
This blog is explaining the process of how it works in the leptin-melanocortin pathway of man. This is what is buried in my Leptin Rx blogs.
THESE IDEAS ALSO EXPLAINED MAMMALIAN ONCOGENESIS
I think this is why the FBI and state medical boards showed up at my doorstep after my TED talk. Amgen knew I had solved the leptin paradox and they wanted to protect their profits. Nature does not make mistakes, but big pharma does. Their game plan can be visualized below.
This also explains why Bill Gates wants to block the sun, buy up farmland, and make sure all his friends at the WEF, WHO, Harvard/Stanford University, and Google help him complete the task of controlling modern humans by controlling their access to light. It is why Nestle wants to control water and make sure all bottled water is fluoridated too. It is why Gates had/has an alliance with Dr. Fauci. He knew Fauci could help him obtain his goals if he could keep people separated and out of the sunlight during a pathogen release. All the pieces fit. Now the global elites control social media so they could limit the discussion on what was evidence-based and what wasn’t and then use cancel culture and state medical boards to police MDs who think in a decentralized fashion.
I was the first MD canceled way back in 2011 because I was telling the truth in my TED talk and on my website. My website has been attacked multiple times by government bot attacks. I’ve been shadow banned on social media and knew it because of my friendship with CEOs in media. I was even thrown off a cruise ship by paleo and Carnivore meatheads. Now you know my journey for humanity over the last 20 years.
This blog now fully explains why Australia leads the world in melanoma creation. They block UV light as national pride while they live on a desert with crap water.
Most modern melanoma treatments have no effect on the tumor’s high recurrence rate. However, when the tumor responds to a non-toxic remedy with decreased emissions, the agent will likely improve the patient’s condition and may even promote a cure. Rather than killing tumor cells, the beneficial agent appears to stimulate the normal cells to overcome the cancerous ones.

WHAT ALTERS the band gap to create the UV light cells need?
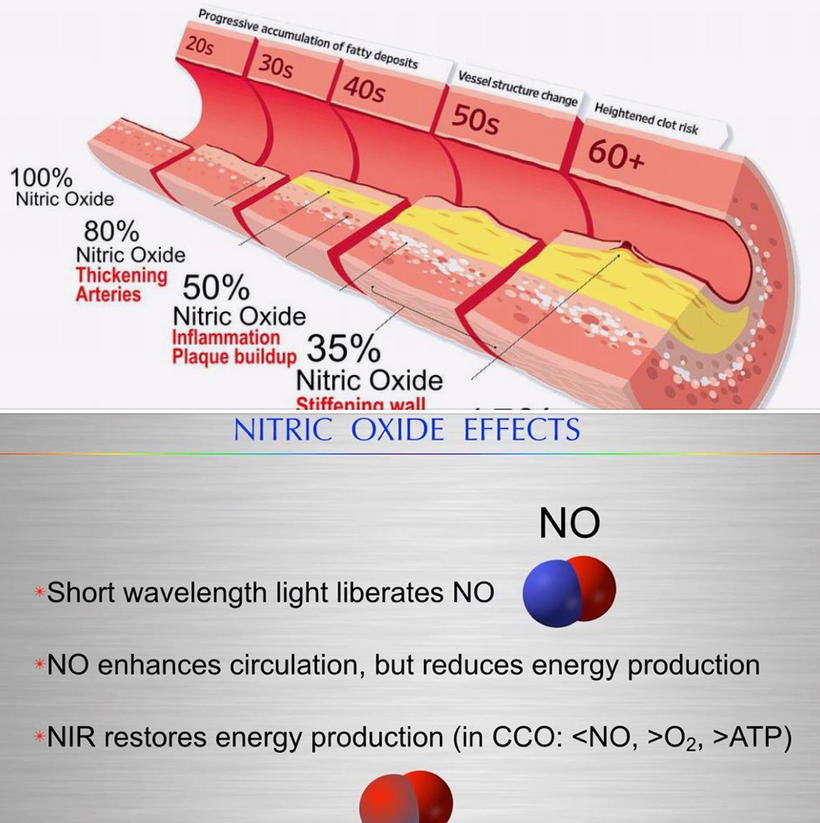
Sunlight via the creation of NAD+, oxygen tensions, the state of the chromophore from a redox standpoint, the oxidation of Iron, the NO levels in tissues, the light frequencies at the skin level, or changes in the atoms that dope the semiconductor. Temperature changes also do it. This occurs in the sleep cycle every night. Those are a few things that can do it. What controls all these variables in a cell? The circadian mechanism. It acts like optical tweezers on atoms and demands precision to keep the AMO organization in the cell organized just as photolithography does in a semiconduction plant.
When the melanosome reached a depth in their tissues where the UV signal was recaptured those cells stayed in this environment and multiplied. Mammals essentially invented metastasis to survive. This metastasis does not come with a negative connotation as metastasis does in cancer today. It carries a positive connotation and refers to retained mobility (neuroplasticity) in your melanosomes that allowed your deep ancestors to survive an asteroid event that blocked sunlight. Vitiligo is present today and is a signal that melanin renovation deep inside our skull is defective for some reason and the organism has lost VUV-IR-A light creation inside. This tells us mitosis is blocked in the central melanin system. The brain recruits melanin from the skin when UV light is absent from the surface of the skin and POMC cannot be made. If POMC cannot be made neither can alpha-MSH. When the cleavage products are sparse many diseases show up in humans.

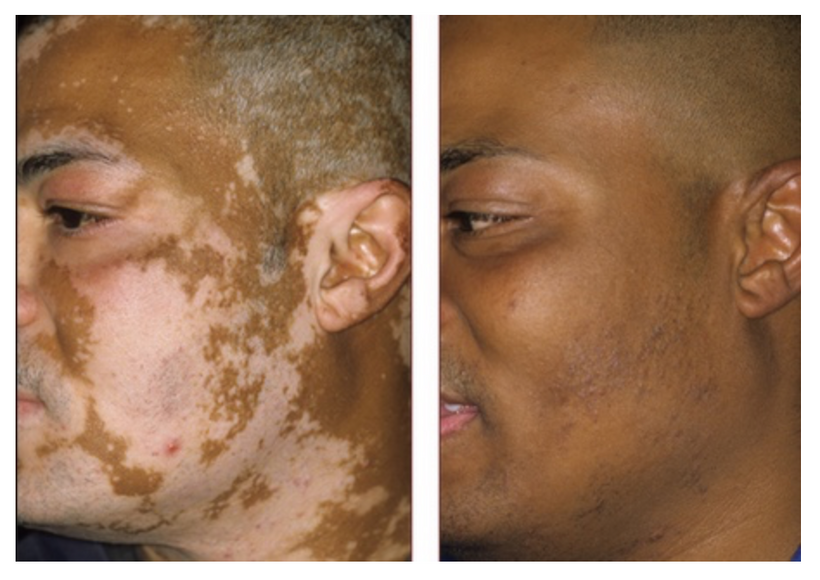
The lady above came to see me 15 years ago with this problem after her dermatologist was not successful in treating her. Over a period of 18 months, her face went from what you see on the left to what you see on the right. It did not take as long as I thought it would. Immediately I began asking all my dermatologist friends to send me their worse cases of vitiligo so I could see how long it would take to re-pigment their skin. Every single difficult case they sent me also had other neolithic diseases associated with the POMC protein. The patients with diseases linked to neuroectoderm responded badly initially to my treatment, but with solar persistence, they all got better, eventually. Also notice how grey her hair was on the left. Her hair lost all of its greys in this transformation too.
My TED talk was about this very issue here in the blog. Within 2 weeks of my TED talk, the FBI and state medical board visited me and the talk was banned. Big Pharma was a major supporter of this TED event. I mentioned this to Dr. Huberman and Rick Rubin on the podcast. Huberman’s response was illuminating for me.
Why did I do this mitohack on the lady above? I’d love to tell you it was altruism in figuring out where most disease comes from, but it wasn’t. I realized that figuring out how fast it took humans to repopulate their skin with melanocytes today, with UV light would give me an idea of how long photosynthesis was interrupted in the mammals and therapod dinosaurs that made it through the KT event.
I did this mitohack when I was in my 18 months of disbelief of realizing everything I had learned in medicine for the first 40 years of my life was essentially a lie. It was before I had written the CT 4-6 blogs and before I wrote the Leptin Rx on paper. I wanted to know how mammals survived this and came to thrive after this event. I wanted to explain the process of what happened in the book, “The Monk Who Sold His Ferrari”

When I looked at the modern situation of man and thought about what ancient mammals faced at the KT event, at the foot of the Michelangelo statue of David, I had my revelatory thoughts about the leptin-melanocortin pathways in mammals. You are currently reading those thoughts in the last few blogs in a way you have never seen them presented before.
The implications of what I just showed you above tell you why mammals live longer too. The positive aspects of metastasis have been kept by evolution in our immune system. All WBC cells today in every mammal contain POMC and are able to block their own emission of UV light to interrupt their own ability to get into mitosis. This allows them to be mobile in the circulatory system to enter tissues to fight pathogens. When they arrive at the pathogen, the cell uses the pathogen’s massive UV light show to turn on the WBC production of POMC and destroy the pathogen. UV light mediates their mobility. This story is laid out in the book below.

Modern WBCs still do what the ancient mammals’ melanocytes did for their neuroectoderm. We retain this ability. You have to know mitogenic radiation to realize the implications in this blog. I have for 20 years now. This is why I created the two slides below.

POMC-related opioid peptides have been found in the immune cells of many vertebrates and non-vertebrates (references in (Stein and Machelska, 2011)). Expression of full-length transcripts encoding all three POMC exons has been found in rats and human leukocytes. This POMC transcript is spliced in the same way as the pituitary transcript and contains the sequence for the signal peptide, which is necessary for the correct routing into the regulated secretory pathway. The POMC protein is also proteolytically processed in a way consistent with the pituitary gland. The production of POMC transcripts is stimulated by various immune and inflammatory mediators (reviewed in (Stein and Machelska, 2011)).
KEY BLOG LESSON: I realized that I might understand really where the cancer epidemic began and why modern centralized medicine has made it worse. Every single Big Pharma chemotherapeutic drug is designed to destroy cells in mitosis. This is the key signal cells use to halt migration. Modern metastasis happens in humans because they no longer can make internal UV light from their melanin semiconductors. When the light signal is lost mitosis stops and the cancerous cell can migrate to another tissue where the UV stimulus is present. That is what cancer really is at its core. It is cells looking for UV light.
I also realized that malignant melanoma is not what we think either. Melanoma might be melanocytes inside of us at deep locations that have had their mitosis interrupted by the degradation of melanin semiconductive proteins and this causes cells to migrate back to the skin to get UV light signals to proliferate once again.
I was stunned by this insight below this masterpiece of a statue. It took me seeing perfection in human form, with sunlight coming through the dome above, while a bird was sitting on the ledge as I looked up. It all clicked in a moment. That moment has never ended for me. It has fueled my passion ever since. I no longer had time for any scientific half-truth.
Modern science has made a career out of mutilating this story. My job is to end it.
SUMMARY
Cancer, obesity, diabetes, and autoimmunity are all related to methylation changes of POMC mediated by light and leptin levels in humans. It is not intuitive because no one has thought about how light controls the levers in the central retinal tract anterior to the RPE, pituitary, and hypothalamus.
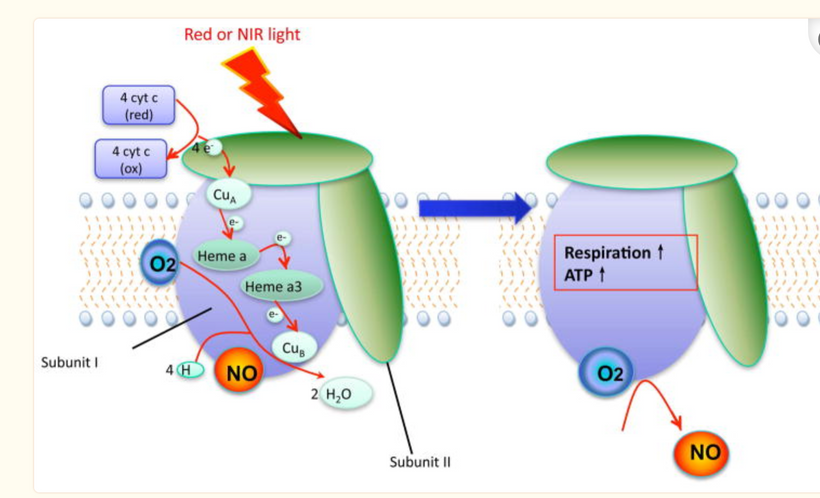
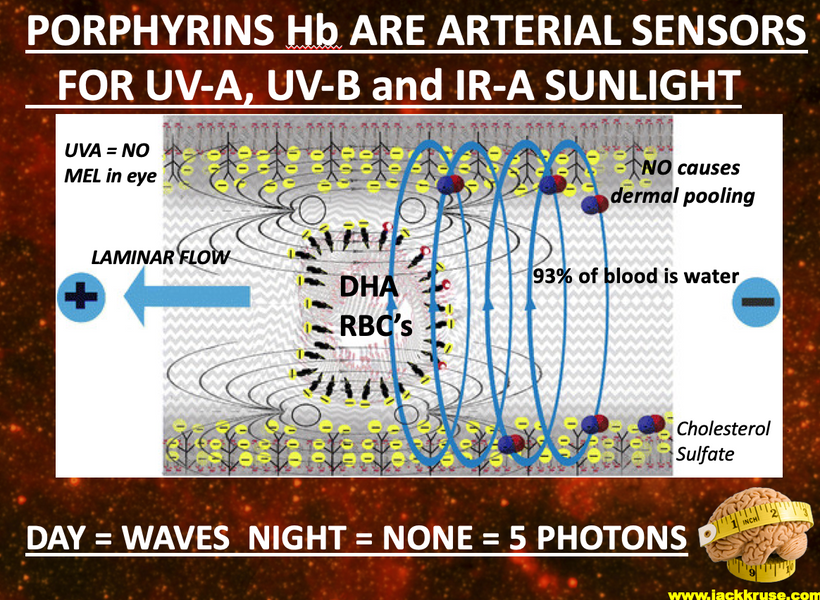
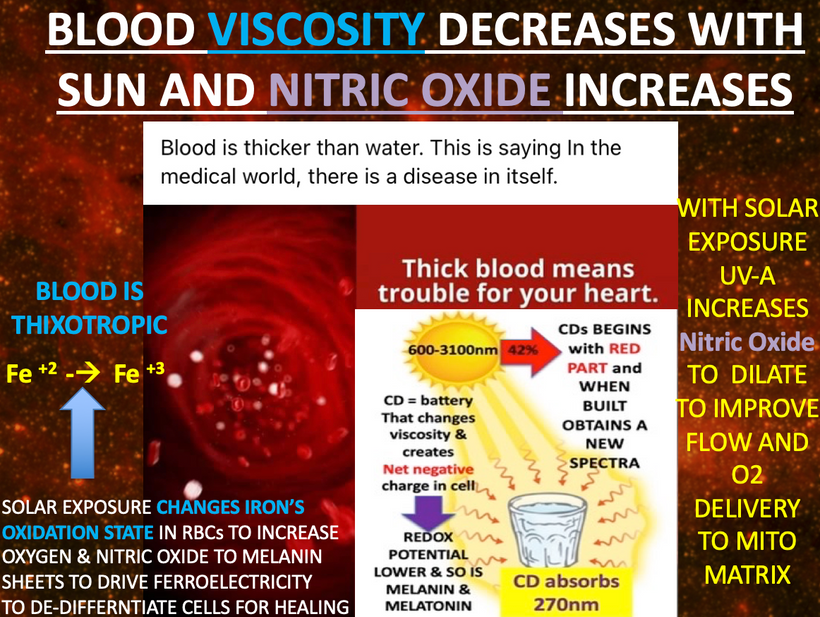
In all mammals, α-melanocyte-stimulating hormone (α-MSH) is a major anorexigenic neuropeptide that thins animals. This tells us tanning skin and weight loss are linked in some way. That way is via light. UV light creates vitamin D which binds to the VDR on the inner mitochondrial membrane on mitochondrial to slow electron chain flow. Slowing electron flow means the animal will eat less food. This slowing is augmented by nitric oxide release at mitochondria at cytochrome c oxidase. NO release is also stimulated by UVA light on our skin and by changing the oxidation state of hemoglobin from +2 to +3 so oxygen and NO are delivered to mitochondria while electron flows are slowed. α-MSH is another post-transcriptional cleavage product of proopiomelanocortin (POMC).
POMC is expressed in the hypothalamic arcuate nucleus (ARC) and is axonally transported to the paraventricular nucleus (PVN), where it binds to the melanocortin 4 receptor (MC4-R). When this happens it augments and decreases food intake and body weight while restoring internal stores of melanin in the brainstem distally.
The hypothalamic expression of POMC is regulated mainly by peripheral circulating leptin. Circulating leptin is affected by our light environment via melanopsin destruction. This liberates Vitamin A from the opsin which in turn becomes a wrecking ball that leads to lowered dopamine, melatonin, and melanin via alpha MSH, and DHA levels in the local tissues. Blue light fattens humans because it raises blood sugar and insulin together.
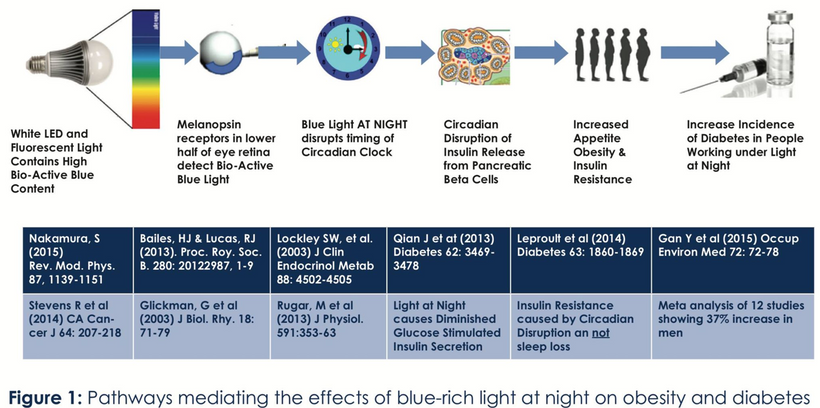
Mediated by the hypothalamic leptin receptor, leptin binding to Pomc neurons activates a signal transduction mechanism that includes activation and binding of several transcription factors [e.g., specificity protein 1 (Sp1), nuclear factor κ-light-chain-enhancer of activated B cells (Nf-kB)] to the Pomcpromoter (10,–12). It was found that in common forms of obesity, both animals and humans become leptin resistant, a phenomenon associated with impaired regulation of energy homeostasis
Adrenocorticotropic hormone (ACTH) is synthesized as part of the precursor proopiomelanocortin (POMC). Therefore it represents a challenge to endocrinologists in understanding how ACTH is cleaved from the precursor to produce the peptide that acts on the adrenal gland to stimulate the release of adrenal steroids.
Modern centralized endocrinology does not focus on POMC or ACTH in humans. The Black Swan should be able to describe the structure, expression, and regulation of the POMC gene, with emphasis on the difference between POMC in the pituitary and POMC in other tissues and in tumors.
Neurosurgeons deal with pituitary tumors. Most people do not know abnormal methylation of POMC is associated with pituitary tumors and with obesity. POMC expression is retrained by promoter methylation [43]. On the other hand, the POMC gene is intrinsically activated in ACTH-dependent Cushing’s syndrome. This disorder may be a consequence of the activation of the highly tissue-specific POMC promoter in pituitary and non-pituitary sites. This promoter contains a CpG island, which is methylated in normal non-expressing tissues but is specifically demethylated in expressing tissues and tumors [24]. Methylation near the response element for the tissue-specific POMC activator PTX1 abolishes the binding of this transcription factor that plays a key role in pituitary development. The POMC promoter could show different degrees of methylation in POMC-expressing hypothalamic neurons, thus influencing food intake and obesity (Figure 10.8).
Secondly, the mitochondriac needs to understand information on alpha MSH and ACTH and their related peptides in the context of the structure of the precursor, how it is processed, and the biological activity of the different peptides derived from POMC. It is important to understand which peptides are present in the circulation and how differential processing of POMC in various organs/tissues produces an alternative spectrum of peptides (including precursors and fragments) in different tissues. This is critical in understanding tissue-level biology from a quantum perspective.
POMC is a prohormone that gives rise to several biologically active peptides that are expressed primarily in the pituitary and brain. This isn’t limited to those tissues. Sunlight in the visible spectra (UV) stimulates POMC with the help of the TSH.
In people who live under fake light, the majority do not make POMC and this means mitosis is defective in their cells. When mitosis is defective in mammals their cells do not divide and they become mobile and invade other tissues. Mammals innovated this process to survive the KT event using their neuroectoderm to help them. Today modern light is the human KT event for the same reason. In fact, People with hypothyroidism struggle to make enough POMC and this makes their skin strophic.
The hypothalamic-pituitary-adrenal (HPA) axis is well recognized for its role in the homeostatic mechanisms in man because it regulates the stress response and the light stress reactions.
The hypothalamic secretion of corticotropin-releasing hormone (CRH) stimulates ACTH synthesis and release from the anterior pituitary, which in turn regulates the synthesis of glucocorticoids in the adrenal cortex where melanin is abundantly found. The impact of the host of factors and mechanisms known to regulate ACTH and related peptides has to be considered in the context of quantum biological activity both at the adrenal level and in other tissues or massive errors will ensue. Modern centralized medicine is expert in creating these errors daily in clinics and hospitals.
Reject centralization.
Become fully decentralized.