The Cosmic Dance of Iron: A Story of Life, Stars, and Evolution
Imagine a universe where the same forces that forge stars also shape the delicate biology of life on Earth. At the heart of this cosmic tale is iron, a humble element with an outsized role in both the fiery cores of dying stars and the pulsing vitality of living cells. To understand iron’s place in biology and evolution, let’s embark on a journey that weaves together the physics of stars, the chemistry of life, and the challenges of our modern world, a story crafted for those curious about the intricate dance of nature.

Act 1: Iron in the Stars
Long before Earth existed, stars were born, lived, and died in a spectacular cycle. Stars begin their lives fusing hydrogen into helium, releasing the radiant energy we see as sunlight. As a star ages and exhausts its hydrogen, it turns to heavier elements, helium fuses into carbon, then neon, oxygen, silicon, and finally iron. Iron is the endgame for a star. Its dense, stable nucleus resists further fusion, halting the star’s energy production. As iron accumulates in the core, the star collapses under its own gravity, triggering a supernova, a cataclysmic explosion that scatters iron and other elements across the cosmos.
This stellar lifecycle mirrors a profound truth: iron marks both the death of a star and the birth of new possibilities. The iron forged in those ancient explosions seeded the universe, eventually finding its way into the rocks of a young Earth and the biology of the life that would emerge. Iron, then, is a bridge between the cosmic and the cellular, a fractal pattern connecting the grand scale of the universe to the microscopic machinery of life.
Act 2: Iron and the Dawn of Life
Fast-forward to Earth, 2.4 billion years ago, during the Great Oxygenation Event (GOE). The planet was a very different place; oxygen was scarce, and life was simple, anaerobic, and bathed in sunlight. Then, cyanobacteria began harnessing sunlight for photosynthesis, releasing oxygen as a byproduct of this process. This “oxygen holocaust” was a crisis for early life, as oxygen’s reactive nature was toxic to many organisms. Yet, from this chaos, a new order emerged. Survivors evolved to use oxygen’s electrical properties, and iron became their key ally.
Iron’s ability to switch between oxidation states (Fe²⁺ to Fe³⁺) made it indispensable. Oxygen, the only paramagnetic elemental gas, is drawn to magnetic fields and alters electrical resistance when it binds to iron-containing proteins like hemoglobin or cytochrome c. This “paramagnetic switch” allowed life to harness oxygen for energy production in mitochondria, the powerhouses of eukaryotic cells. The tricarboxylic acid (TCA) cycle, with oxygen as its final electron acceptor, became the engine of complex life, fueling everything from single-celled organisms to the human brain, which consumes 20% of our body’s energy to drive its “Ferrari engine.”
Iron’s role didn’t stop at energy. It became a cofactor in enzymes that synthesize neurotransmitters, ensuring that our brains could communicate effectively. It is embedded in heme proteins, such as hemoglobin, to transport oxygen through our blood. However, iron’s power comes with a catch: it reacts with oxygen to produce reactive oxygen species (ROS), such as hydroxyl radicals, which can damage cells through processes like lipid peroxidation. Life evolved to balance the benefits and risks of iron, storing it safely in proteins like ferritin and relying on sunlight’s red and ultraviolet frequencies to optimize mitochondrial function and protect against oxidative stress.
Act 3: Iron in the Human Brain
In humans, iron is a double-edged sword, especially in the brain. It’s essential for energy production in mitochondria and for synthesizing neurotransmitters like dopamine, which governs movement and reward. Iron accumulates naturally in the brain as we age, particularly in regions like the basal ganglia, globus pallidus, and substantia nigra. These areas, rich in gray matter, hold two to four times more iron than white matter, where myelin insulates nerve fibers.
But when iron accumulates excessively, trouble brews. In neurodegenerative diseases such as Parkinson’s, Alzheimer’s, and Friedreich’s ataxia, iron overload in neurons triggers oxidative stress, resulting in the production of reactive oxygen species (ROS) that damage lipids, proteins, and DNA. This process, known as ferroptosis, is particularly devastating in the retina, where photoreceptors and intrinsically photosensitive retinal ganglion cells (ipRGCs) are particularly vulnerable. These cells, which detect light to regulate our circadian rhythms, rely on iron-containing proteins and melanin, a pigment that chelates iron to protect against oxidative damage. When this balance falters, ferroptosis destroys neurons, disrupting circadian signaling and contributing to diseases such as Parkinson’s, where the loss of dopamine-producing neurons in the substantia nigra is linked to iron overload and reactive oxygen species (ROS).
Why does iron accumulate in sick or dying neurons? The answer echoes what is found in dying stars. Just as a star amasses iron in its core as it runs out of energy, neurons hoard iron when their energy production falters, often due to mitochondrial dysfunction. In both cases, iron signals a system on the brink, teetering between stability and collapse.
Act 4: Iron, Light, and the Modern World
Now, let’s bring this story to the present. Iron’s role in biology evolved under the influence of natural sunlight, with its balanced spectrum of red, ultraviolet, and blue light. Morning sunlight, rich in red and UV, optimizes mitochondrial function and protects against oxygen toxicity. But modern life has disrupted this ancient rhythm. Artificial blue light from screens and LEDs, which dominates our evenings, photoexcites iron in heme proteins and melanin, shifting iron from Fe²⁺ to Fe³⁺. This generates local magnetic field changes and amplifies ROS production, driving ferroptosis in neurons and photoreceptors. It also does something else. It breaks a fundamental law of physics as you’ll soon see.
In the retina, blue light-induced iron overload impairs rods, cones, and ipRGCs, disrupting circadian rhythms and the myelination process, the process that insulates nerve fibers. This connects to broader neurological damage, as seen in Parkinson’s, where neuromelanin’s protective iron-chelating role turns pathological under blue light stress. The link between Parkinson’s and melanoma, a skin cancer associated with melanin dysfunction, further highlights how iron and light interact across systems, with blue light increasing reactive oxygen species (ROS) in both the brain and skin.
Even in the oceans, iron plays a protective role for life when you look for the evidence. Marine life, from plankton to fish, relies on iron to support the food chain. Some hypothesize that iron in seawater absorbs excessive electromagnetic fields (EMF) from human technologies, shielding marine ecosystems much like iron in a star’s core absorbs energy before its collapse. This parallel suggests that iron’s role in biology is deeply tied to electromagnetic forces, a connection we’re only beginning to understand. It turns out the reason why iron is so important ties the weak force in the cosmos. The cosmos weak force why homochirality evolved as it did on this planet.
Act 5: Iron’s actions in Evolution
Blue light (400–500 nm) is not a passive environmental cue; it is the modern chiral stress agent that directly erodes local parity violation (PV) asymmetry in melanated tissues by exploiting the melanin–iron complex.
Blue Light → Fe³⁺ → Fe²⁺ Redox Flip
Blue photons photoexcite the porphyrin-like centres in melanin and neighboring heme proteins, driving a one-electron reduction of ferric (Fe³⁺) to ferrous (Fe²⁺) iron. This liberates nitric oxide (NO) from nitrosyl complexes and creates a localized hypoxic signal identical to the one used in stem-cell niches. This allows iron to carry oxygen later in the GOE.
Melanin–Iron Synergy Amplifies ROS and Racemization
Melanin is never iron-free in vivo. Iron-saturated eumelanin exhibits dramatically broadened near-IR absorption and a shifted transient absorption spectrum (BJSTR 2024). When blue light strikes this complex. What happens when this occurs?
Superoxide (O₂⁻) and H₂O₂ production skyrockets via cyclic one-electron transfer. Should I remind here that catalase quenches superoxide and it too is a heme protein? You see how life was organized by the GOE now?
Mitochondrial ROS is not the villain; it is the ancient GOE-evolved redox signalling currency. The real danger has always been uncontrolled Fenton chemistry driven by free or poorly ligated Fe²⁺, which converts benign H₂O₂ into DNA-shredding •OH radicals. Evolution spent 2.4 billion years of the GOE building an exquisite system to prevent exactly this, and modern light/environmental mismatches are dismantling it piece by piece.
Act 6: Iron’s Lesson for Humanity
ALAS1 resides in the mitochondrial matrix for a reason. It is the light-controlled iron gate.
When sunrise red/IR hits the retina → melanopsin → SCN → hepatic sympathetic axis → ALAS1 transcription peaks at ZT10 → heme peaks ZT12 → Rev-Erbα represses gluconeogenesis and sequesters iron safely into heme. This is why sunlight reduces glucose so well. It also explains why all forms of diabetes are light diseases.

Iron’s story is one of balance between energy and destruction, creation and collapse. In stars, iron marks the end of a lifecycle, scattering elements to birth new worlds. In life, iron powers our cells, but it demands careful regulation to prevent oxidative damage. The Great Oxygenation Event taught life to harness iron and oxygen under the guidance of sunlight, but modern humans have strayed from this balance. Our reliance on artificial light and EMF disrupts the delicate balance of iron, contributing to a chronic disease epidemic.
For those learning about biology and evolution, iron offers a profound lesson: life is a fractal of the cosmos, governed by the same forces that shape stars. To thrive, we must respect these ancient rhythms, embracing natural light, minimizing artificial nnEMF, and supporting our mitochondria. Iron, the element that links stars to neurons, reminds us that we are not separate from the universe but a continuation of its story.
Key Takeaways for Learners
Iron’s Cosmic Origin: Forged in dying stars, iron seeded Earth and became essential for life.
Iron in Biology: It powers energy production in mitochondria and neurotransmitter synthesis, but can generate toxic ROS if unregulated.
Mitochondrial heme protein containing iron like CCO are literally the GOE survival technology: an oxygen-handling photo-bioelectrical machine that doubled as the executioner when oxygen got out of control in cells.
Light and Iron: Natural sunlight balances iron’s oxidative states, whereas artificial blue light disrupts this balance, potentially driving all chronic diseases.
Evolution’s Lesson: The Great Oxygenation Event shows how life adapted to oxygen and iron, a balance modern humans must rediscover to combat chronic disease.
By understanding iron’s role, we glimpse the unity of life and the cosmos, a story written in stardust, sustained by sunlight, and now at risk in our electrified world.

SUMMARY
The role of Iron should be thought of as a universal energy mediator of energy flow.
Iron’s ability to switch between oxidation states (Fe²⁺ to Fe³⁺) makes it a cornerstone of energy transfer across scales:
In Stars: Iron accumulation in a star’s core signals its end. As fusion slows, iron absorbs energy, emitting chaotic EMF before a supernova—a process driven by energy loss.
On Mars: Mars’ core cooled 4.1 billion years ago, halting its magnetic dynamo. Solar wind stripped its atmosphere, leaving iron oxides on the surface, a testament to it history of energy depletion.
In Life on Earth: During the GOE, cyanobacteria used iron to harness oxygen, a paramagnetic gas, for energy production in mitochondria. Iron in heme proteins (e.g., hemoglobin, cytochrome C oxidase) facilitates oxygen transport and electron transfer in the tricarboxylic & urea cycles.
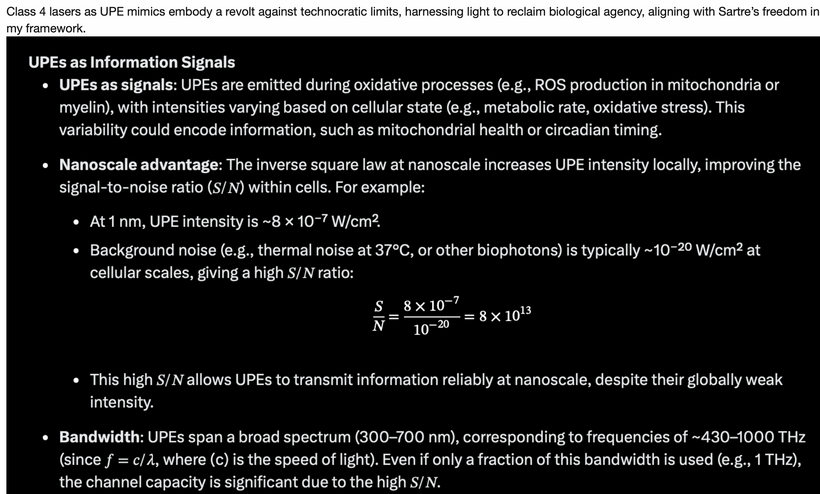
However, iron’s reactivity produces reactive oxygen species (ROS), which emit UPEs; biophotons that carry quantum information. This was the biggest development in evolutionary history ON EARTH because it set the stage for eukaryotic evolution to occur soon after heme proteins evolved on Earth. Every single node in the ferroptosis pathway is a heme-containing, red/UV-absorbing, spin-selective protein that evolved under the GOE’s oxygen + UV-A pressures. The heliosphere-Earth-life system is a fractal energy transfer network and the last step in evolution of heme protein protection schema for oxygen was ferroptosis.
Disrupting one level (e.g., magnetic field or electric fields of the sun or dark) cascades to others, draining energy and driving systemic collapse.
Trees, humans, and marine life show stress via altered UPEs release, that leads to altered methylation patterns, and oxygen utilization. In humans, this manifests as leptin resistance, circadian disruption, and chronic diseases.
DARPA’s use of light to communicate, or its venomous bioweapon injection program, EPA’s geoengineering, and FCC’s EMF policies exacerbate this energy drain, mimicking Mars’ historical fate 4.1 billion years ago.
If you want your tissues to avoid Mars’ fate, humanity must realign with natural energy cycles built during the GOE. What are they?
1. Minimize nnEMF: Reduce exposure to artificial EMF to restore UPE fidelity and mitochondrial coherence.
- Harness Sunlight: Use natural sunlight (250–3100 nm) to optimize mitochondrial function and leptin signaling via the efferent loop of light.
- Protect the Magnetosphere: By addressing solar weakening and cosmic radiation through your own personal awareness of the laws of physics that determines how iron really operates in your body. It is not what you’ve been told.
The heliosphere, Earth, and life are wirelessly connected through a fractal energy system. Iron biology, is a key mediator of energy and information, underscores the congruence between a dying star, a dead planet, and a diseased human. By understanding and respecting these cycles, we can harness the same forces that forged stars to sustain life on Earth. The information driving this was no a planet, it was the interaction of the electromagnetic and weak forces in the cosmos.
The more we bathe cells in man-made electromagnetic fields (light), the more we force them to re-enact the Great Oxygenation Event, but without 2 billion years to evolve a proper defense mechanism. That was the take home lesson of this slide in Vermont 2017.

The mitochondrial matrix is the original iron prison built during the GOE to prevent oxygen + iron from annihilating life.
It later added protection schemata using melanin because of how melanin and iron weave together.
Blue light and nnEMF are the modern jailbreakers of the entire system.
Red light, DDW, and proper circadian timing are the ancient wardens keeping iron working to protect us from oxygen toxicity.
CITES
- Sekiguchi, M., Hayakawa, M., et al. (2006). Evidence on a link between the intensity of Schumann resonance and global surface temperature. Ann. Geophys.
- Global Research. (2019). The Weakening of Earth’s Magnetic Field Has Greatly Accelerated.
- INRAE Study. Frontiers in Ecology and the Environment. Trees as early warning systems for volcanic eruptions.
- Toilekis, Z. PhD Thesis. Structure of leptin receptor related with obesity.
- Helliwell, R. A. (1975). Power line effects on the magnetosphere.
- Kazemi et al. (2013); Trewavas (2014); Deshayes et al. (2024). Studies on biophoton coherence in trees and humans.