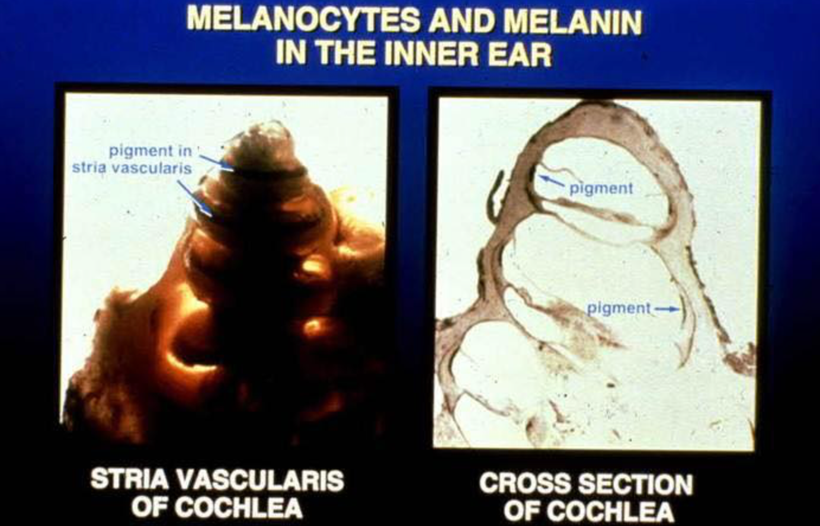
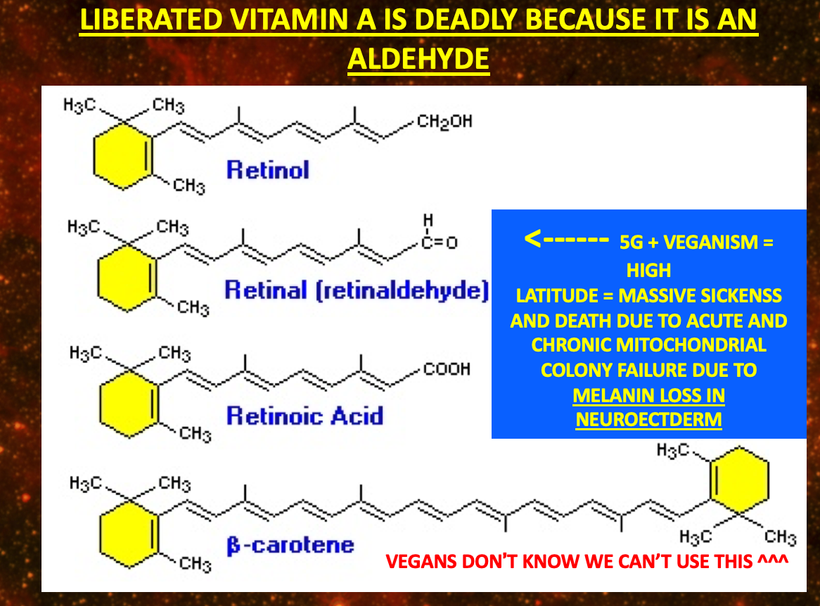
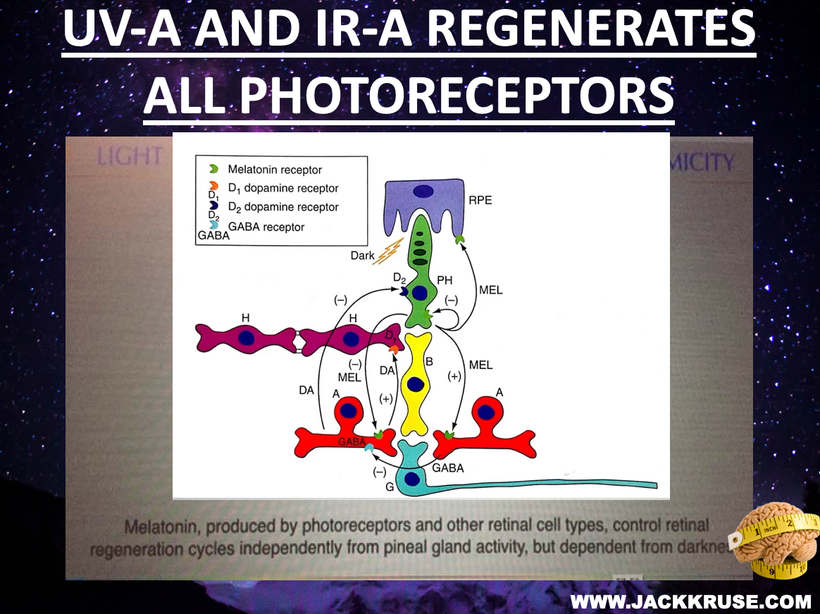
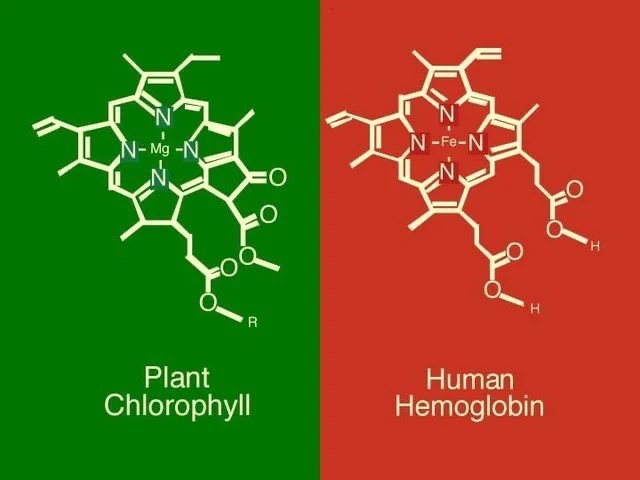
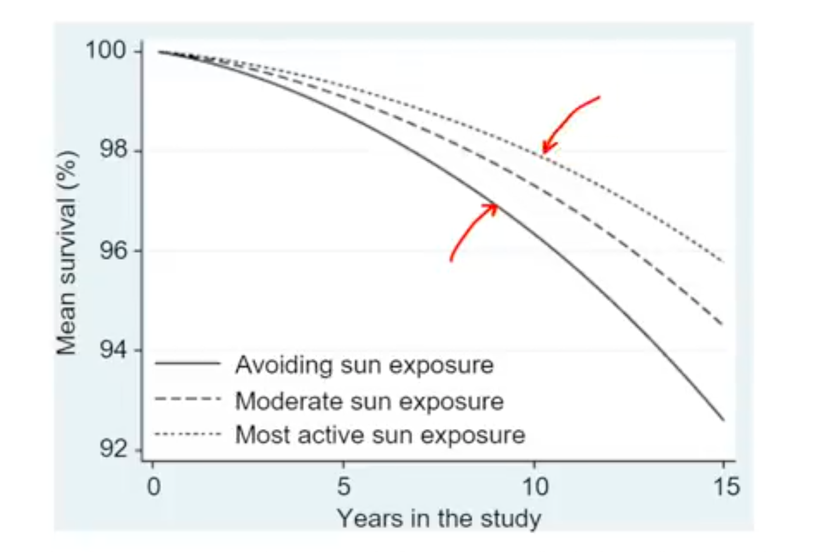
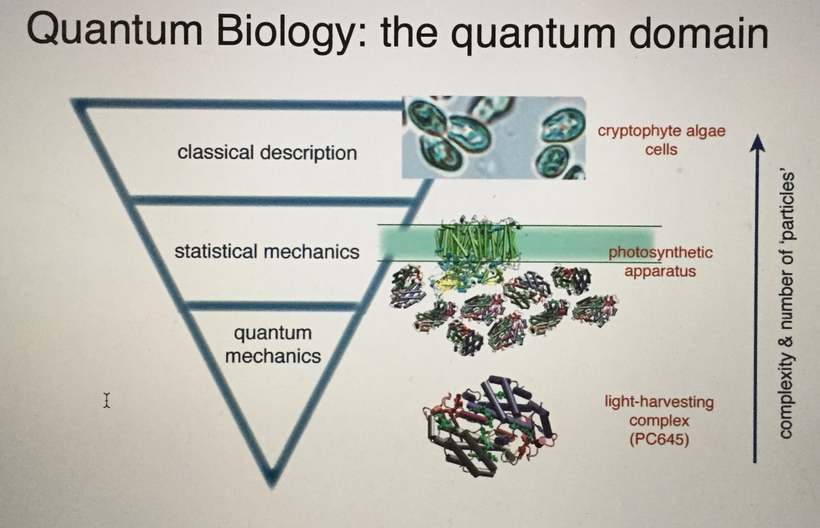
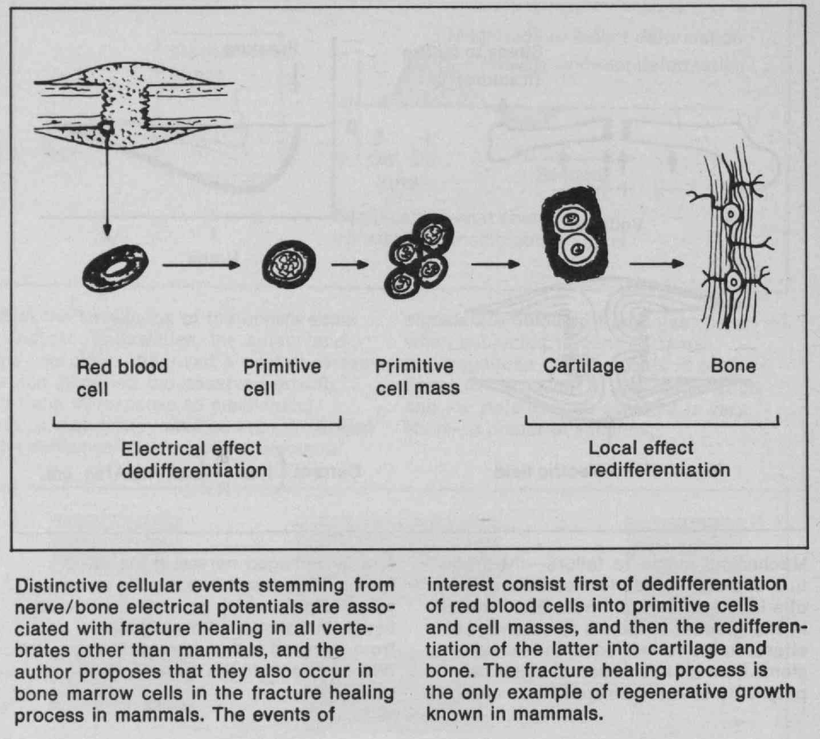
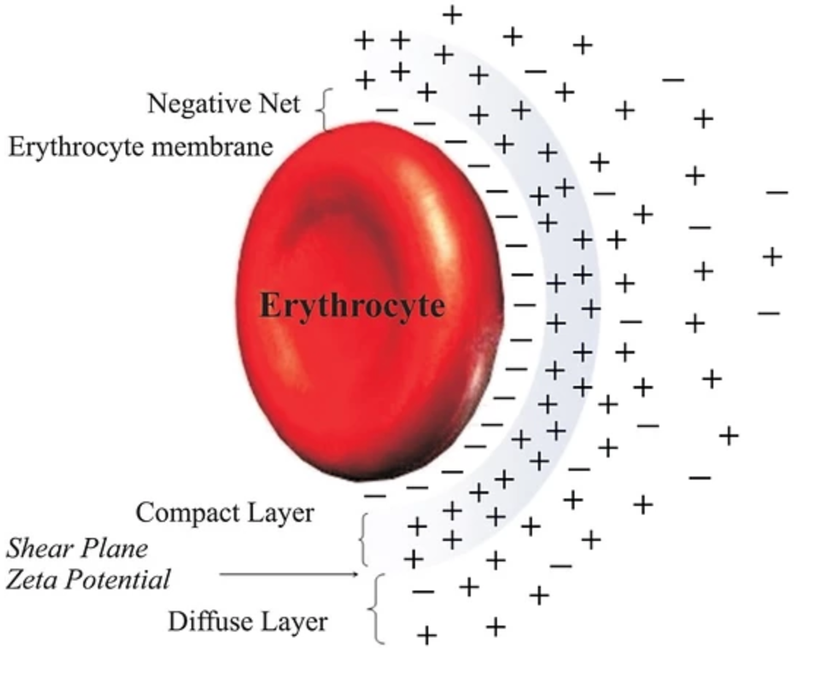
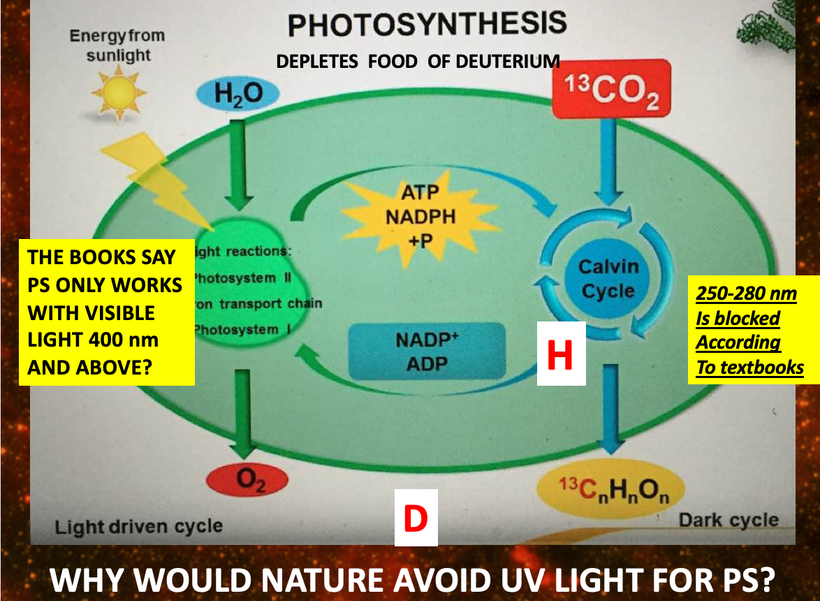
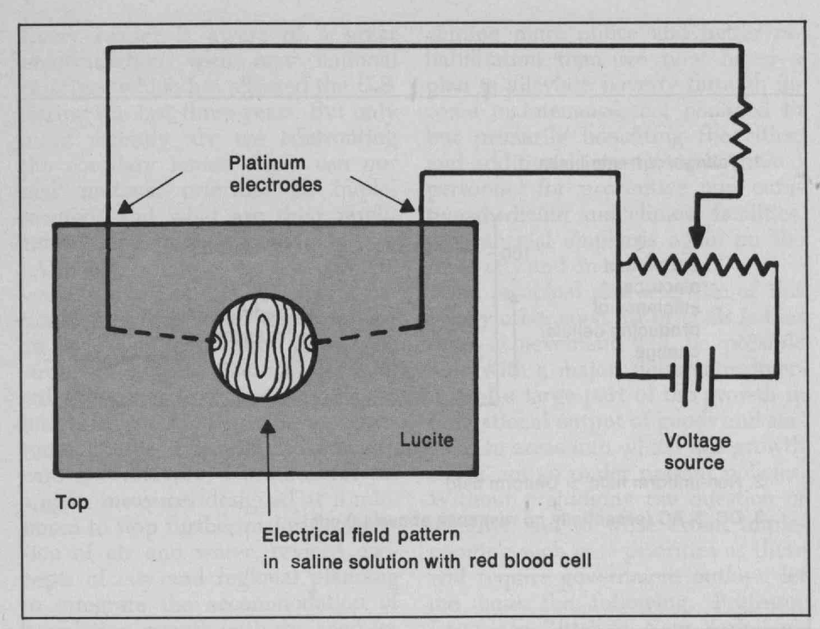
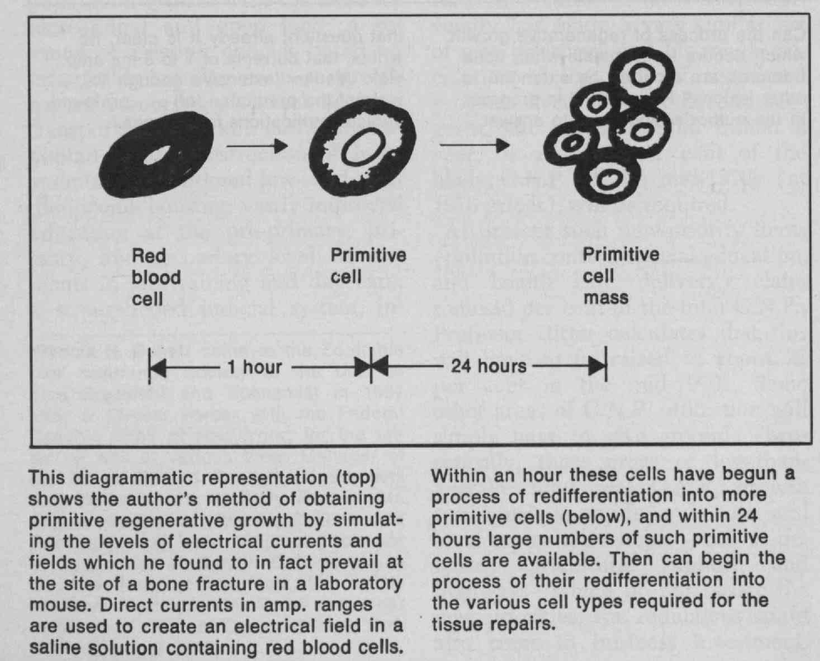
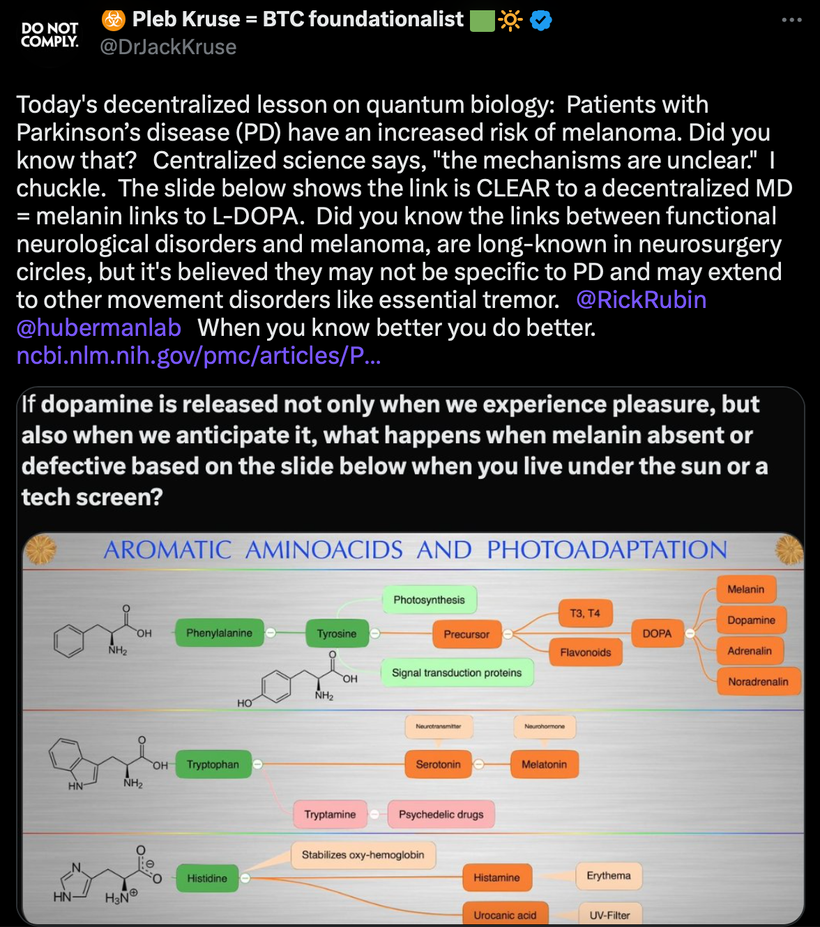
There has been some recent news out about folate levels, sunlight and artificial light. Many people do not know folate is a photosynthetic chemical and it links dark skin (melanin) for natural folate protection.
We have clear evidence (Cite 1) that ultraviolet radiation affects directly in proportion to folate human blood. Seasonal cycles repeat annually and light stability is more common at low latitudes. Therefore, the amount of photosynthetic chemicals we use acts as a photosensitizer to our chromophore proteins. This has big implications inside the tropics. The percentage of low folate values increases in summer by almost 3.5 percent in comparison to winter. Moreover, geneder differences are huge. Overall folate levels are lower in men as compared to women, regardless of seasonality. This makes sense because of who has the children.
Vitamin B9 (Folate) was first extracted from spinach leaves in 1941. The term folate comes from the same root as foliage: green and leafy. Folates are vital: they accept carbon atoms and pass them on as needed as the fundamental basis for proper methylation. This implies that during summer months folate levels should be expected to be at their lowest levels NATURALLY.
Folate biology has a specific and tight seasonal control mechanism linked to light. This is not a food story at all

What if there is no seasonal or light controls in place?
Folate is often given to pregnant women to prevent spinal dysraphisms today. This is something pediatric neurosurgeons deal with and why I know a lot about them. The incidence of these conditions has dropped but it is has done at a COST. Why? but too much folate/folic acid causes cognitive haze and sleep difficulty and may cause neural migration problems in artificially lit environments. This should awaken you what I wrote in Quantum Engineering #45.. In North America, (CAN/USA) folic acid was added to all grains in 1996. This is only a few decades ago and we are seeing the transgenerational effects of this right now in our children’s disease phenotypes.

In general, it does not appear that even large amounts of folic acid taken orally are acutely toxic in adults. However, given the fundamental role of folate levels in synthesizing nucleotides (including RNA and DNA) and in methylation reactions as a methyl donor, high levels may have inadvertent implications for proper methylation of DNA during times of rapid cell division, such as in prenatal development. You’d think the idea that adding folic acid to the food supply might have caused centralized medicine to expect unintended consequences. Few thought about, much less studied it. I have always been worried about this effect since I learned that B12 was a photoreceptor in humans. (slide from Vermont)

This idea has been speculated in research circles as early as 2005, and specifically speculated to be relevant for the increase in autism in 2011.
MOST PEOPLE WITH MTHFR DEFECTS ARE SUPERSENSITIVE TO LIGHT VARIATIONS
An early review of potential problems with mass folic acid supplementation of the food supply was undertaken by Lucock and Yates. Here, they noted that a drastic increase in folates could lead to a selection for the previously rare MTHFR genetic substitution of T for C at area 677 (MTHFR C677T), and that if folic acid is supplemented at doses above 400 mcg that unmetabolized folic acid will circulate in the blood supply at a level largely consistent with the excess dose. In 2005, Lucock and Yates noted that high levels of folic acid in the blood does not generally occur as a result of ingesting natural folates and that “no work has been done so far to evaluate the biological and genetic consequences of excess long term exposure” to these circulating folic acids on DNA/RNA biology. After that review, there were two separate findings of unexpected increases in asthma and breathing problems associated with folic acid use.
It now appears that we have clear data that excessive methyl donor transfer has significant epigenetic effects in humans. This work dovetailed with another review questioning the wisdom of mass folic acid supplementation published in 1996. Smith et al. pointed out that by supplementing the food supply; several hundred thousands of persons are exposed to greatly increased levels of folic acid. These authors noted that prior research had shown that expectant mothers with low vitamin B-12 (most vegans/vegetarians) AND high levels of folic acid were associated with offspring having an unexpected increased risk for insulin resistance and disease associated with this condition. This is worrisome when you know that blue light exposure does the same thing and more.
Troen et al. found that some women past childbearing age subjected to high folic acid supplementation may be at risk for reduced immune system functioning causing inflammatory autoimmune conditions to spike. This problem has gotten worse since I first looked into it in 2005.

Did you know mammals exhibit genomic instability under conditions of folate deficiency in the skin. Remmber your skin is a neuroectodermal derivative. A lack of folate adversely effects your skin’s cellular capacity to handle UVR light. This is why so make pale gingers burn so fast. Moreover, did you know that optimizing folate levels in skin is beneficial in preventing or repairing the pro-carcinogenic effects of UVR exposure? Folate restriction by any modern excuse leads to rapid depletion of intracellular reduced folates resulting in S-phase growth arrest. Did you know this leads to ncreased levels of inherent DNA damage, and it causes increased uracil misincorporation into DNA. This is called a frame shift mutation and it is how light can cause transepigenetic signaling. This frameshift mutation will not cause a significant loss in overall cellular viability. It is how mammals adapt to changing seasonal light environments. Folate depleted keratinocytes can be sensitized toward UVR induced apoptosis. This changes the biophoton spectra emission from mtDNA and this displays a diminished capacity to remove DNA breaks. This allows for changes in phenotype. This occurs by photo and oxidative DNA alterations. Your dermatologist likes to call this damage, but Mother Nature uses this for seasonal adaptation in your skin. When this occurs more UVR = more melanin production. This protects your folate stores from degrading. Nature is amazing when you see her recipes. Dermatolgyis dangerous because they do not understand what she is doing on your behalf. Thus, folate deficiency creates a “permissive environment for genomic instability to allow for seasonal change. It is not an early event in the process of skin carcinogenesis or autism. If one abuses the use of folic acid and blue light than you can and will get diseases. The effects of folate restriction, even in severely depleted, growth-arrested keratinocytes, were reversible by repletion with folate foods. In summertime, foods with folate are plentiful in the tropics for this reason. Photosynethesis always has our cells backs.
FOLIC ACID SUPPLEMENTS EXACERBATE MTHFR DEFECTS
Methylenetetrahydrofolate reductase (MTHFR) is an enzyme encoded by the MTHFR gene and has significant implications in the field of decentralized medicine. This enzyme plays a critical role in the folate metabolism pathway, which is integral for processes like DNA synthesis and repair, as well as epigenetic alterations via methylation by light variation. Variants of the MTHFR gene are associated with altered enzyme activity, which can, in turn, affect mtDNA heteroplasmy and disease phenotypes. This happens by alterations created in the spectra of biophotons made via metabolism. You’ll hear more about that soon enough.
Increased consumption of folic acid is prevalent in our modern world, and has negative consequences for MTHFR patients.. The effects of folic acid on the liver, the primary organ for folate metabolism, are now being unfolded. Methylenetetrahydrofolate reductase (MTHFR) provides methyl donors for S-adenosylmethionine (SAM) synthesis and methylation reactions.
New data now suggests that high folic acid consumption reduces MTHFR protein and activity levels, creating a pseudo-MTHFR deficiency in the liver and skin of mammals. This deficiency results in hepatocyte and keritinocyte degeneration in both organs, suggesting a 2-hit mechanism whereby mutant hepatocytes cannot accommodate the lipid disturbances (how fatty acid carbon lenghts are dealt with and how melanin operates in the skin) and altered membrane integrity arising from changes in phospholipid/lipid metabolism. People forget the skin biology needs optimal light to control the lipid rafts in the skin as seasons change. Folic acid destroys this ability. This data has clinical implications for individuals consuming high-dose folic acid supplements, particularly those who are MTHFR deficient.
WHAT ABOUT CYP VARIANTS?
CYP enzymes are heme based and subject to blue light toxicity. They have been identified in all kingdoms of life: animals, plants, fungi, protists, bacteria, archaea, and even in viruses. This is why so many astronauts have viral particles in the blood and space is known to foster cataracts and protein folding issues in the eye. However, they are not omnipresent in all bacteria; So space blood infections vary compared to the ones we see on Earth. More than 50,000 distinct CYP proteins are known to man.
Most CYPs require a protein partner to deliver one or more electrons to reduce the iron (and eventually molecular oxygen). Remember electrons must be excited by sunlight to use the photoelectric effect in the photon traps in aromatic amino acids. Based on the nature of the electron transfer proteins, CYPs can be classified into several groups: I covered this with Rohan during a past Sunday Q & A that lasted 5 hours. Members should listen back to those recorded Q & As to refresh their minds.
- Microsomal P450 systems, in which electrons are transferred from NADPH via cytochrome P450 reductase (variously CPR, POR, or CYPOR). Cytochrome b5 (cyb5) can also contribute to reducing power to this system after being reduced by cytochrome b5 reductase (CYB5R).
- Mitochondrial P450 systems, employ adrenodoxin reductase and adrenodoxin to transfer electrons from NADPH to P450.
- Bacterial P450 systems, employ a ferredoxin reductase and a ferredoxin to transfer electrons to P450.
- CYB5R/cyb5/P450 systems, in which both electrons required by the CYP come from cytochrome b5.
- FMN/Fd/P450 systems, originally found in Rhodococcus species, in which an FMN-domain-containing reductase is fused to the CYP. (cytochrome 2 in humans is related to this system.
- P450-only systems, which do not require external reducing power. Notable ones include thromboxane synthase (CYP5) for platelets, prostacyclin synthase (CYP8) for PG synthesis, and CYP74A (allene oxide synthase). You should begin to see just how we are beings of light who need solar programming by sunlight and not man-made light.
Anything transferring electrons to proteins = a semiconductive circuit. When you understand the photoelectric effect only works with photons and electrons you begin to see why SNP status are mostly superfluous and generally do not matter when you’re in the sun chronically and you have melanin in your integument and interior properly renovated.
Melanin operates in us to charge separate water into its components of H+ and oxygen and 2 -4 electrons. The level of oxygen dissolved in cells is critical in varying the ultraweak biophoton signature from metaboilsm. Those two electrons liberated from charge separation obviate the need for exogenous and endogenous sources of electrons (grounding/food). SNPs evolved because mammals varying in how they ground and what they eat based on latitude and what photosynethesis can provide. SNPs and SAPs tell vary in how melanin biology is operating. They are like equalzer buttons for music made in cells.
People with a lack of melanin think SNPs matter way more than they do because functional medicine MDs tell them this. This is incorrect. Pale people without enough melanin lack electrons to run their semiconductive circuits. You’d be wise to avoid that level of centralized thinking in the future. Folic acid lowers electrons = lowers your redox power.
How humans operate is specific to humans and not to other mammals. Most of the BH4 and Vitamin C used as cofactors deliver electrons to the biochemicals. BH4 and Vitamin C deliver the exogenous sources. Melanin delivers the endogenous source of electrons. Humans create massive amounts of electrons when POMC is operational in their tissues. Here you see Noether’s thereom show up yet again.

THE SUN IS THE BEST SOLUTION TO THIS PROBLEM
SUNLIGHT reduces all these risks, while modern lighting exacerbates it. Moreover, it appears nature is trying to tell us that the sun raises melanin and melanin is protective. Strong solar cycles in photosynthesis seem to simultaneously lower folate in foods in the summertime for a deep reason. That reason is epigenetic hypermethylation which can lead to sleep apnea and cancer formations later in life due to altered DNA methyl marking. This process also alters how RBC circadian cycles (ferrodoxin) can work within their circadian cycles with the innate immune system and TOLL receptors. Most people with anemias have this intrinsic problem linked to modern behaviors around light.

Folate is destroyed by strong sunlight with both UVA and UVC light. Darkened skin protects the stores we have, but there is now proof that folate levels are designed to be low when the solar radiation is strong in the local environment. These days most people are eating food humans have been engineered and/or genetically altered in some way. Then add in the effect of Artifiical light day and night. This throws off the normal variation of the natural folate cycle during seasons. Today, people in developed countries are getting MASSIVE amounts of folates in the form of folic acid. This is the real reason to avoid grains. Folates are now being ingested in three ways: as natural folates from food, as synthetic folic acid added to processed grains and synthetic from vitamin supplements. All of these idea are counter evolutionary to Nature’s laws.

SUMMARY
Methylation patterns are linked to how light is transformed in a cell. When sunlight is absent for any reason mitochondrial redox drops. When this happens energy transformation drops. As a result of a lack of energy methylation problems shows up in both genomes. Decentralized clinicians know what to look for. Allopathic and function docs would know what to look for because they still have no idea that light controls the enzymatic flux in metabolic pathways.
Loss of mitochondrial redox power causes methylation problems to manifest in your labs without any SAP/SNP issues present in your MTHFR survey or nuclear genome. Very few clinicians know a lack of sunlight causes methylation defects.
It shows up in your labs in a very specific pattern of results as an accumulation of methionine and S-adenosylhomocysteine, with low or low-normal levels of S-adenosylmethionine (SAM) and homocysteine. PBM treatment fixes it. Sunlight is always the best treatment. Supplements not so good.
As a result of the supplementation of folic acid, the circulating level of unmetabolized folic acid, as well as total folates, has greatly increased over the past generation, probably to levels largely unprecedented in human history.

Folic acid has been shown to be able to epigenetically alter the functioning of the genome and to have long term effects on gene expression as I mentioned above.
The Centers for Disease Control Vaccine Safety Datalink data set compared children with autism to control children on several variables. Many people who think the link of vaccines to autism might be shocked to find out that folic acid supplementation during gestation is associated with a serious increased risk for autism. I believe the combination of artificial light and folic acid supplementation are causing neural migration problems. This is why parental melanin levels are important clues. This effect remains even when health-seeking behaviors and other variables are controlled. This is information parents of kids with AUTISM need to know. Autism, asthma, allergy, ectopy, eczema, diabetes T1D, T2D, and MODY, auto-immunity, and spinal abnormalities have their lowest incidence is lowest in equatorial environments and it appears now we know why this is the case. This is decentralized medicine 101 I will bring to El Salvador.

CITES
1. Valencia-Vera E, Aguilera J, Cobos A, Bernabó JL, Pérez-Valero V, Herrera-Ceballos E.. ‘Association between seasonal serum folate levels and ultraviolet radiation’. ‘J Photochem Photobiol B’. 2019 Jan;190:66-71
2. https://threadreaderapp.com/thread/1656714608793485326?refresh=1683832764
3. https://www.linkedin.com/article/edit/6330012027309875200/