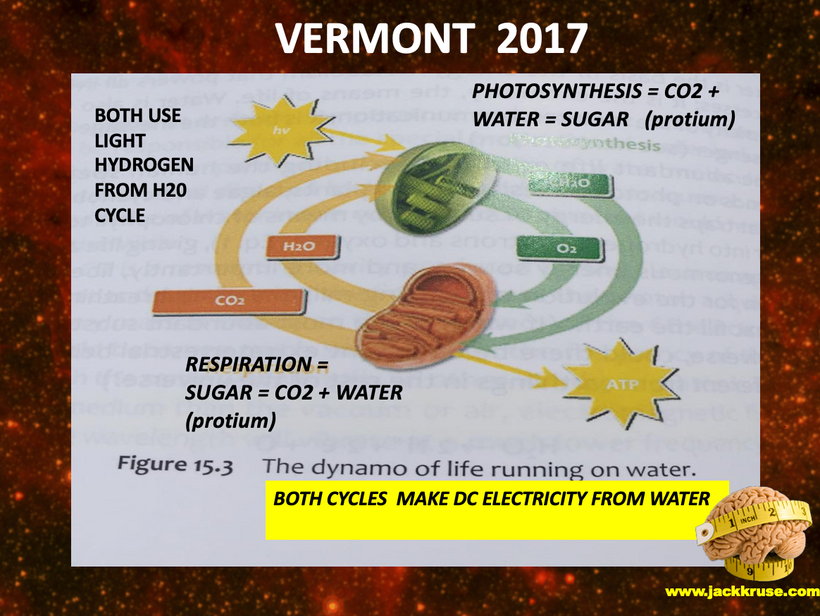
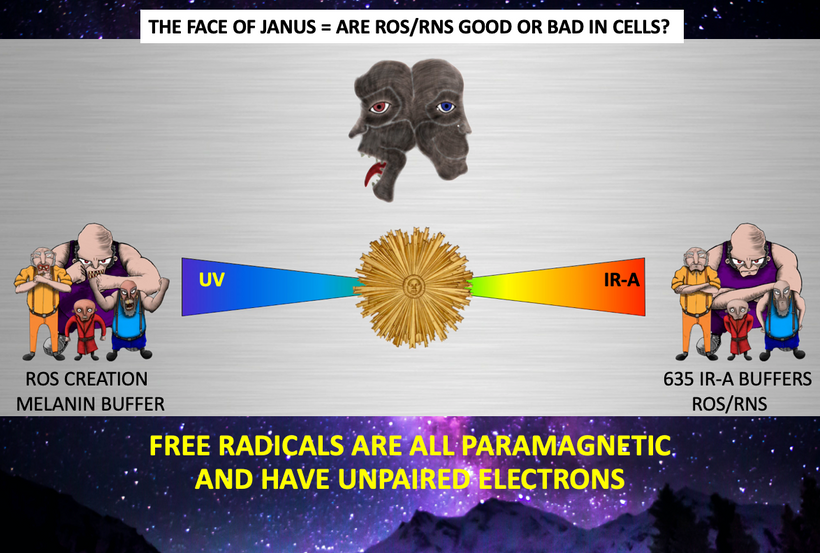
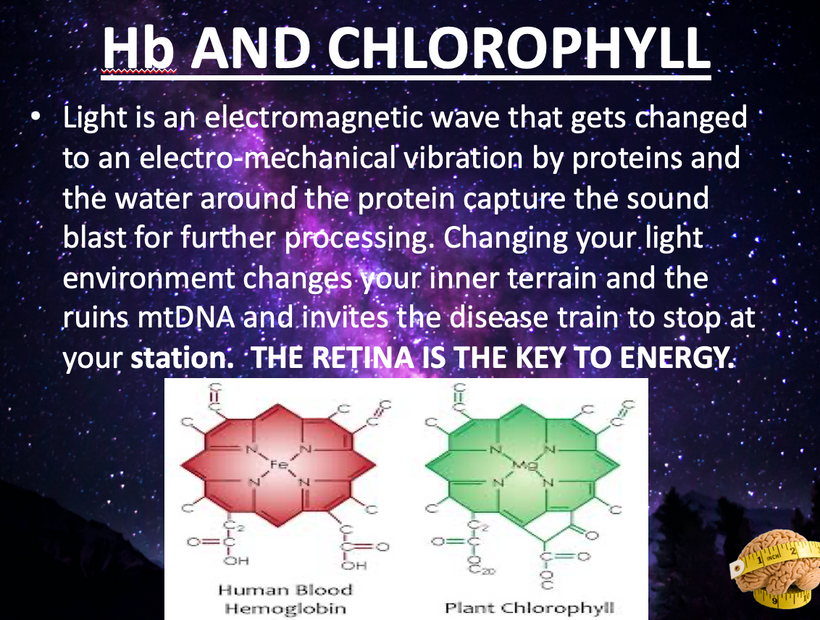
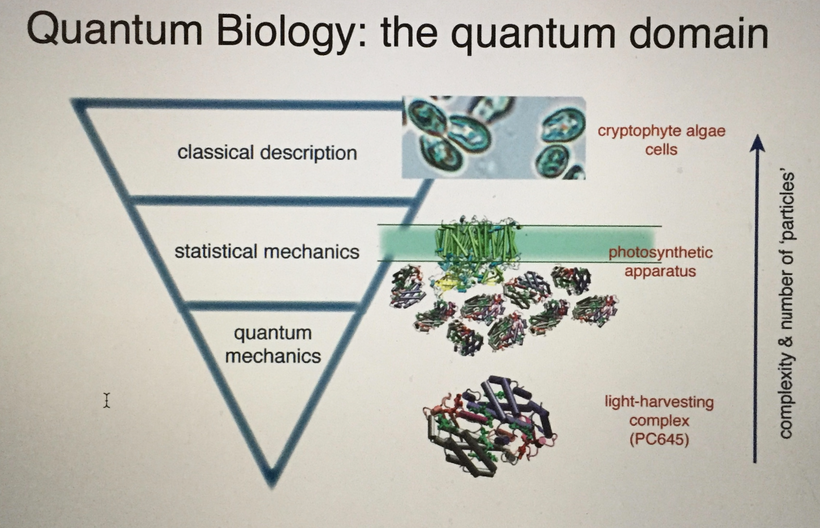
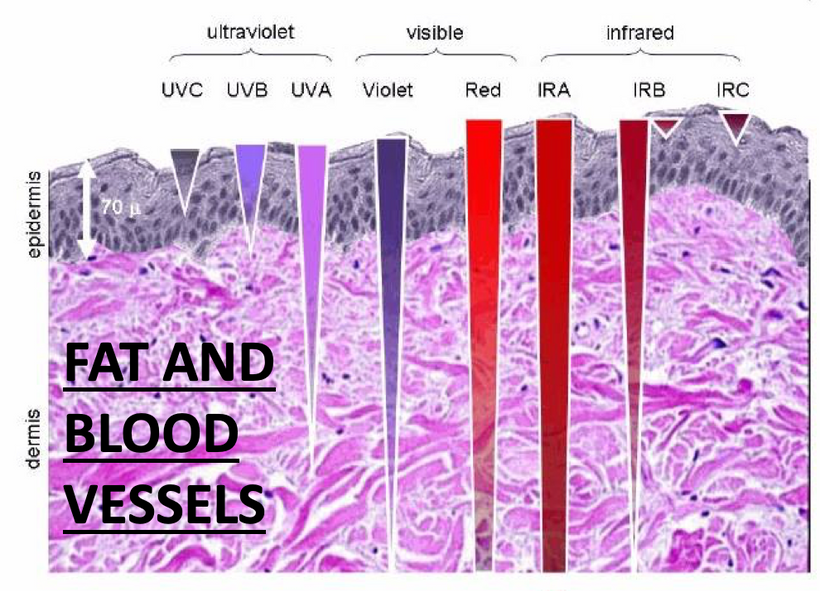
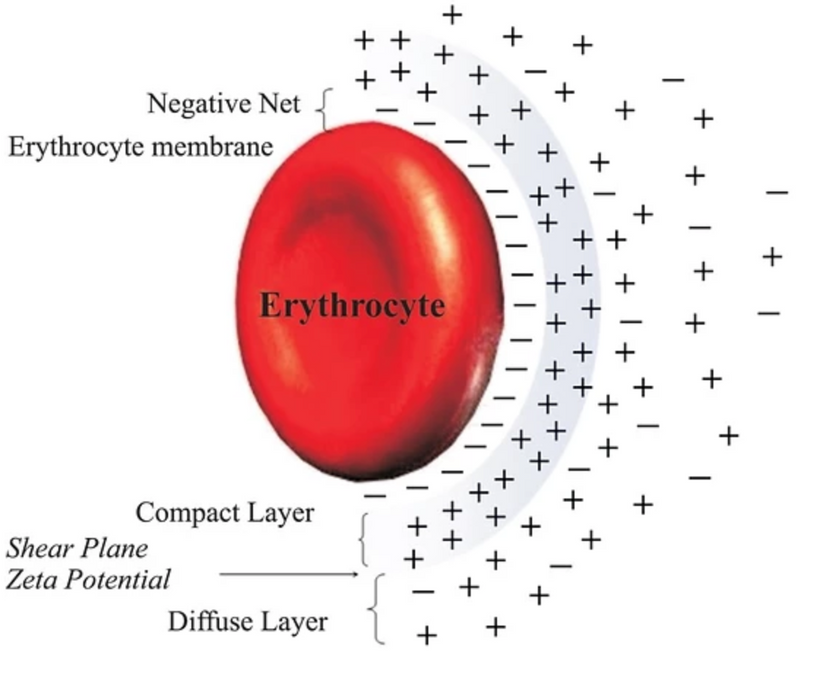
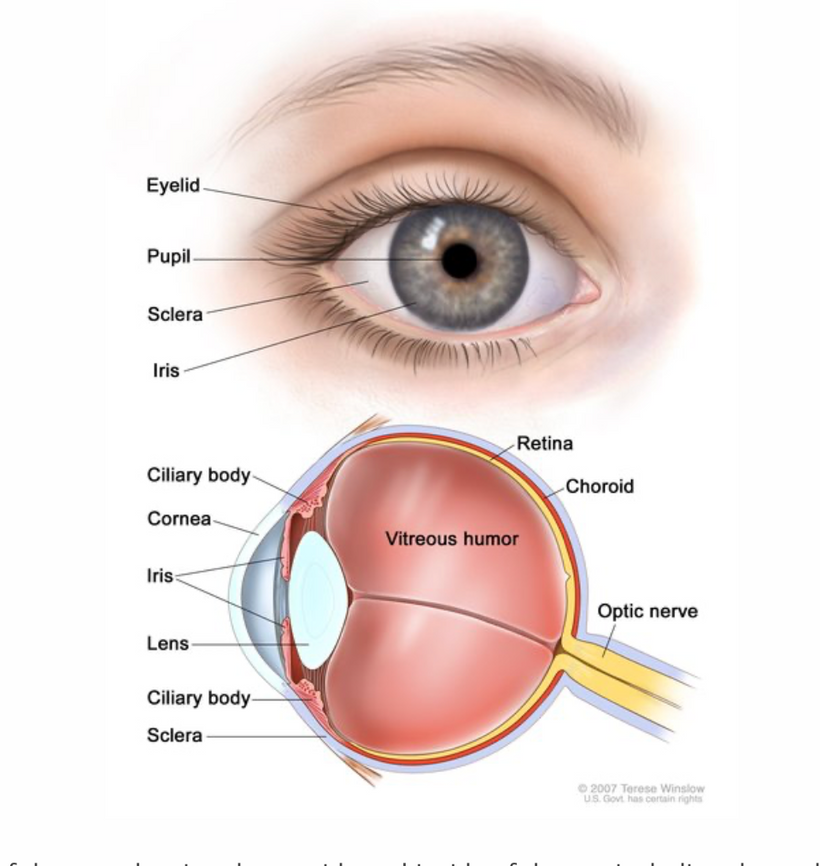
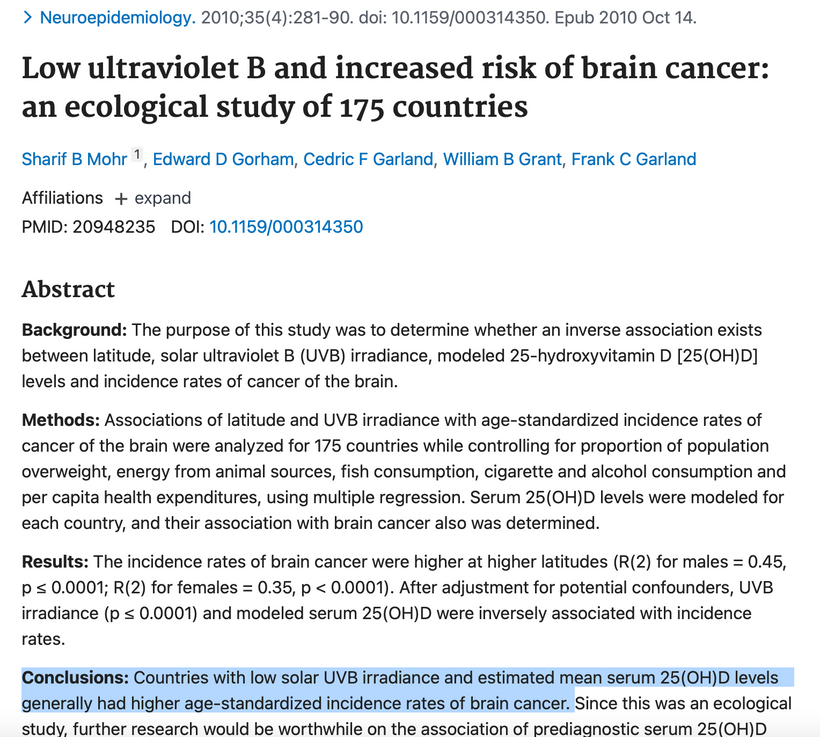
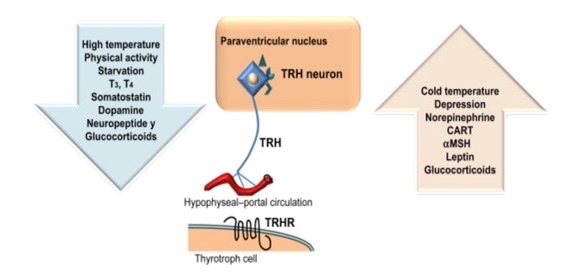
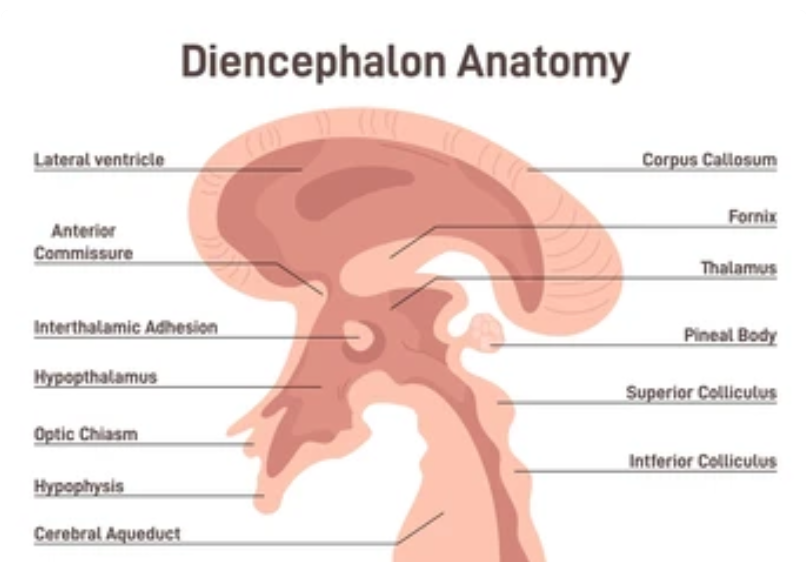
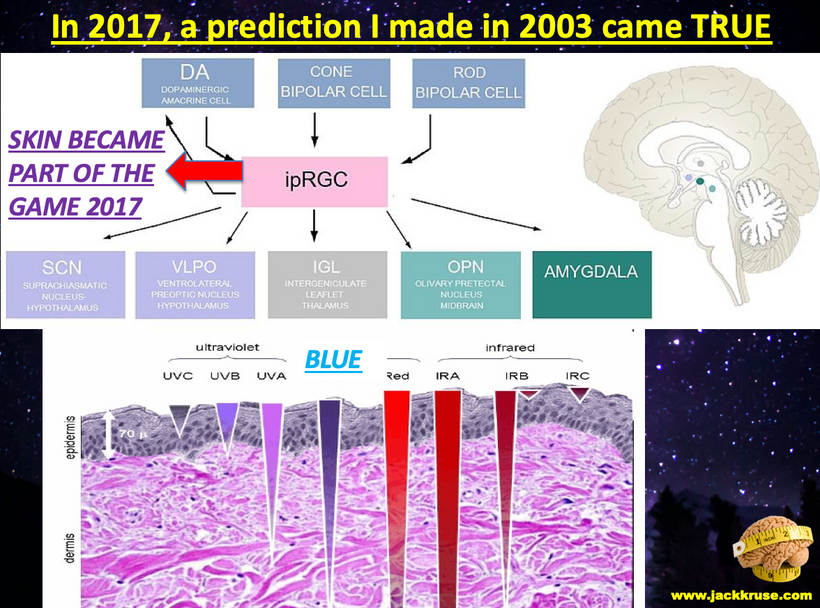
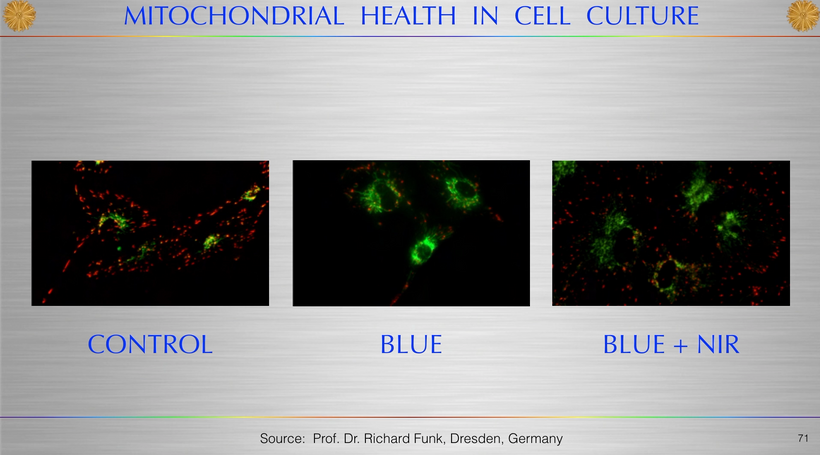
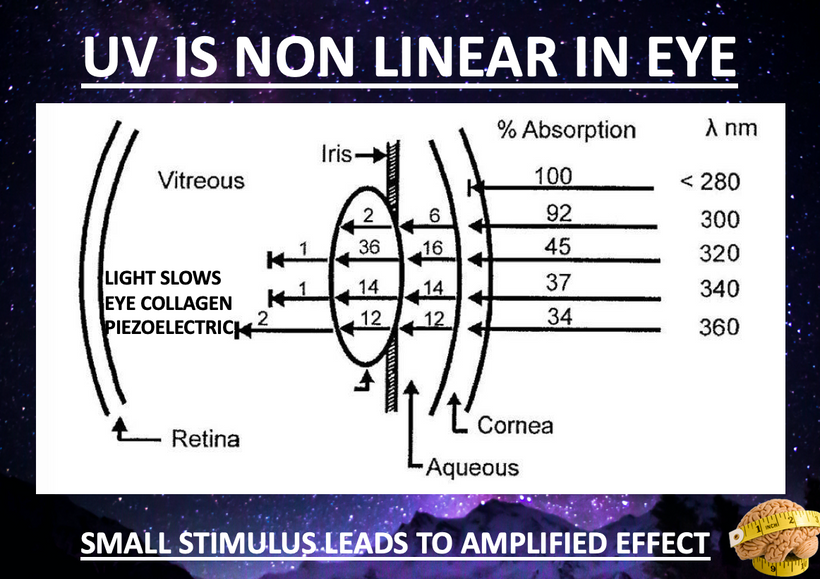
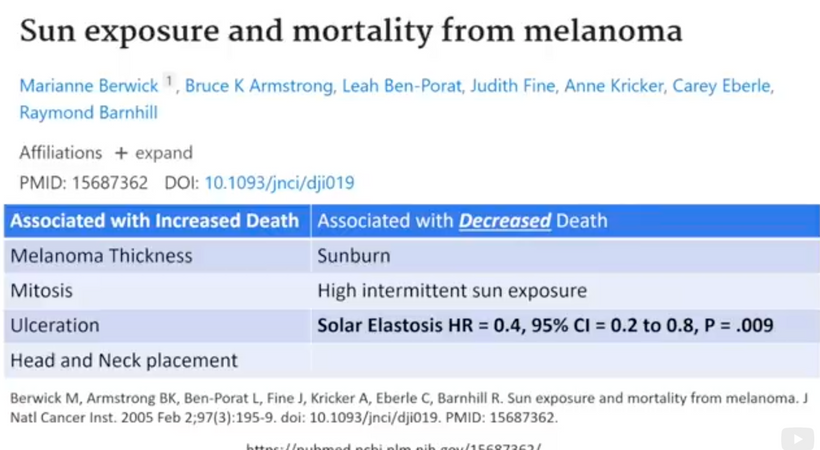
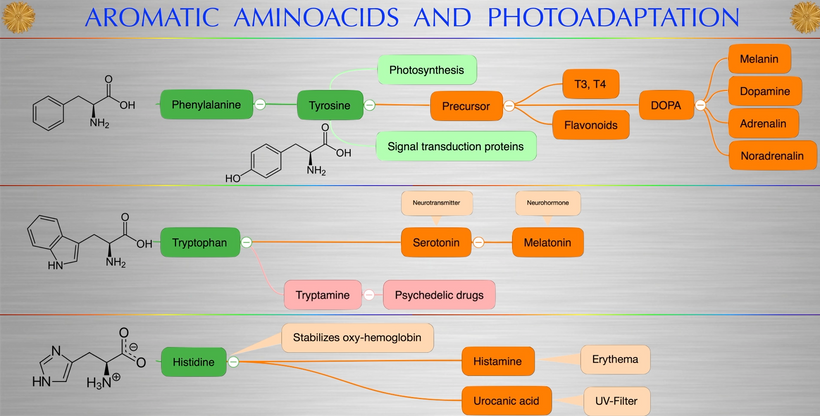
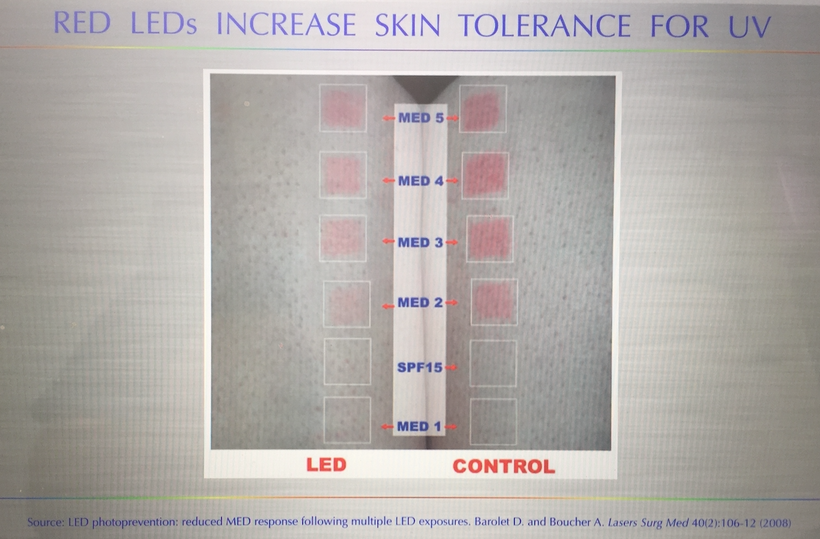
Part one ended by asking you a provocative question…………
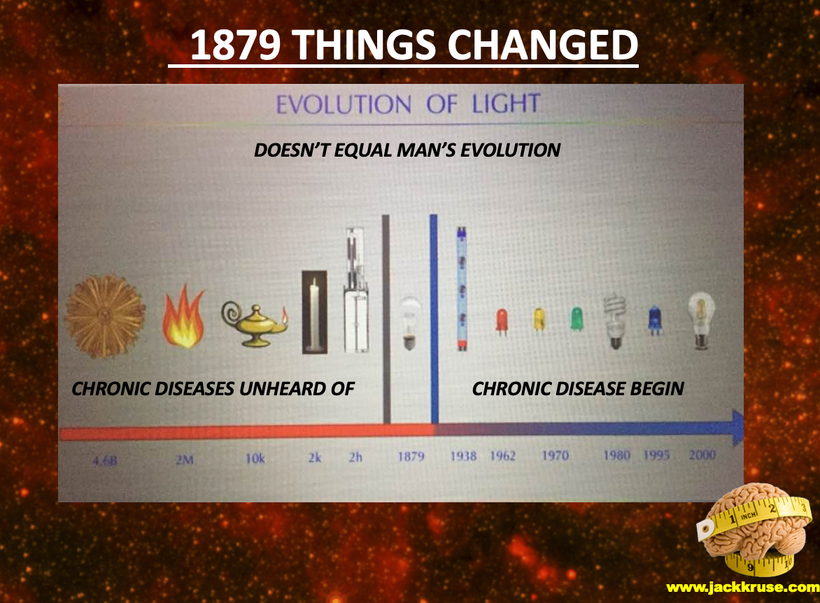
Part two begins with the answer. All life is built around atoms. Atoms were unknown at the time of Darwin. Not even the math equations of Maxwell on light were complete. But Faraday’s work was done in Darwin’s time. It seems Darwin never thought about the sun on his trip in the Beagle on his way to Equador’s Galapagos Islands.

Darwin’s theory on evolution stated in his book stated: “As natural selection acts solely by accumulating slight, successive, favorable variations, it can produce no great or sudden modifications; it can act only by short and slow steps” (Darwin 1859).
Darwin cannot explain 3 things we know are true today
1. Cambrian explosion
2. The transition from a chimp to human
3. Why do primates have the same number of genes as humans yet are so different?
A longstanding debate in evolutionary biology concerns whether species diverge gradually through time or by rapid punctuational bursts at the time of speciation. The theory of punctuated equilibrium states that evolutionary change is characterized by short periods of rapid evolution followed by longer periods of stasis in which no change occurs. Despite years of work seeking evidence for punctuational change in the fossil record, the theory remains contentious. This changed in September 2022 when genomic arrays of the clade of mammals were completed. What did it show?
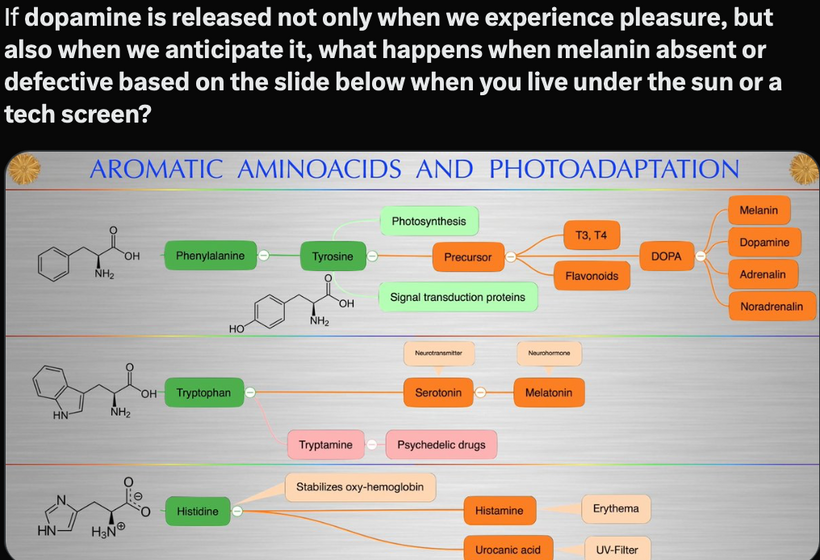
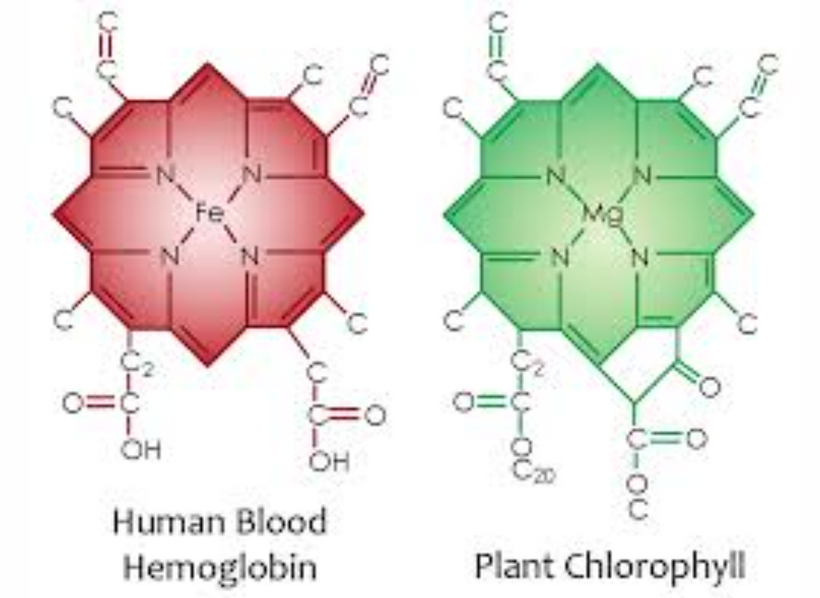
The reason evolutionary biologists were impotent to find these answers in the fossil record was that melanin from the POMC gene explained these paradoxes and was highly conserved in DNA that was stable in the mammalian tree.
See that September 2022 paper here.
https://doi.org/10.1073/pnas.2209139119
Mammalian superpowers were not only having the ability to create sugar from light but they all had the intrinsic property of using melanin to transform light energy into chemical energy (H+) to create ATP. This meant that they did not need a ton of food or oxygen to live. This explains why they were so successful during the age of dinosaurs living underground in poorly lit environments.
Today, we now realize this mammalian superpower was underutilized 65 million years ago by these animals because most of their melanin was external to their body plans. After the KT event, oxygen creation by photosynthetic plants was disrupted for some time. This played right into mammalian survival and dinosaur demise. The lack of UV light in the terrestrial environment allowed for melanin neuroplasticity (metastatic motion) of internalizing melanin from their exteriors into their body plans.
This UV quantum effect allows for future melanin superpowers seen in mammals post-KT event to interact with water in their bodies to create ATP without total reliance on oxygen. When melanin is hydrated and in the presence of full-spectrum light, there is water molecule dissociation and ongoing transforming energy. This meant ATP could be created almost completely from the energy that emanates from the melanin by the charge separation of water inside of cells.
IF SO WHERE IS MELANIN LOCATED IN MAMMALS?
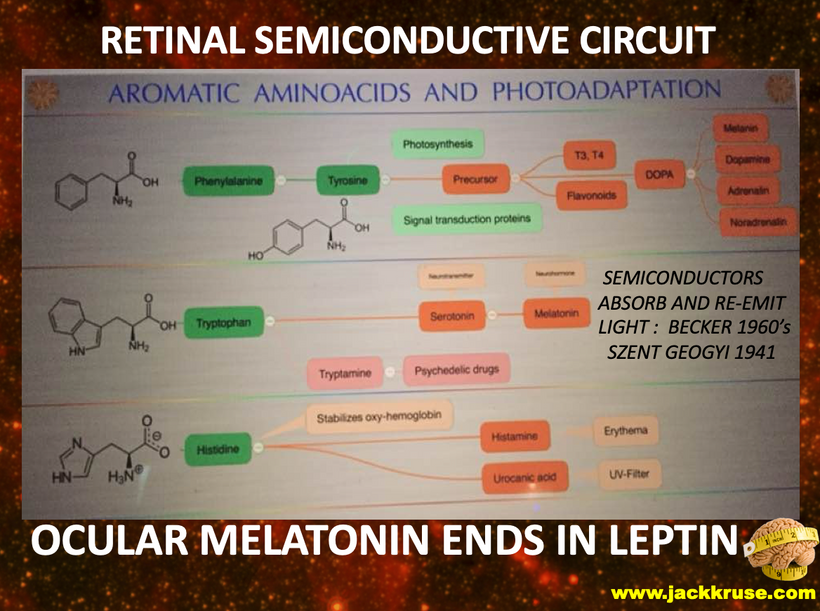
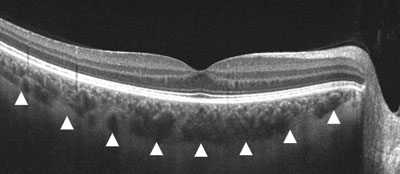
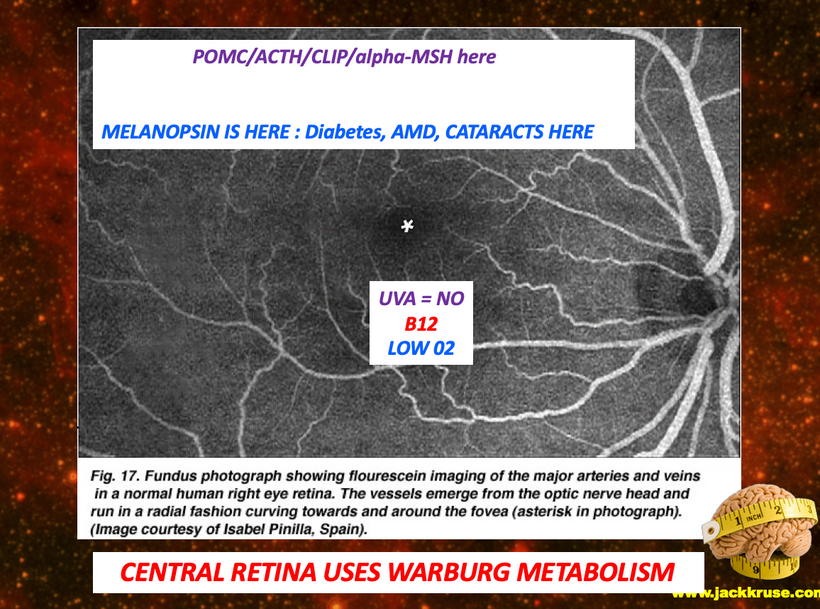
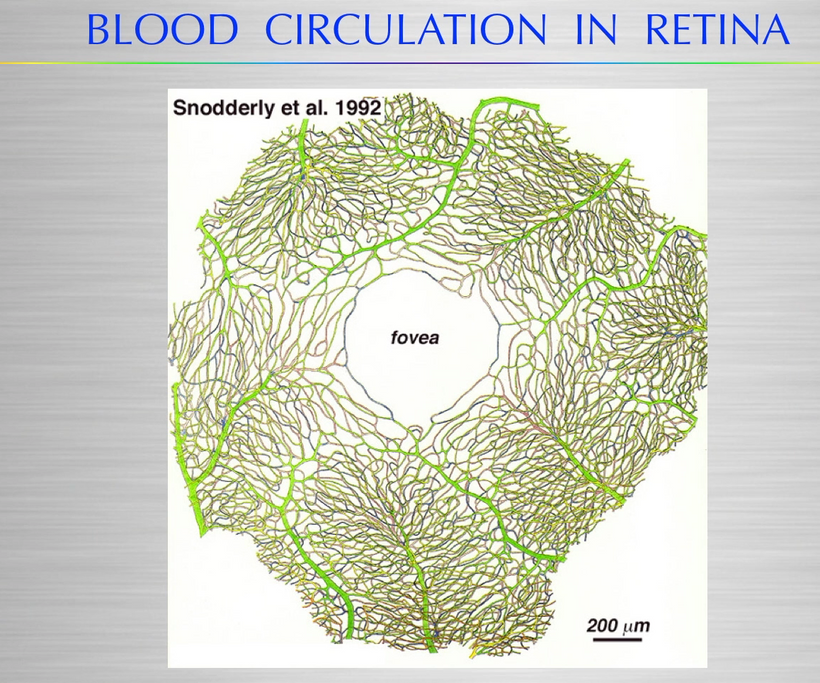
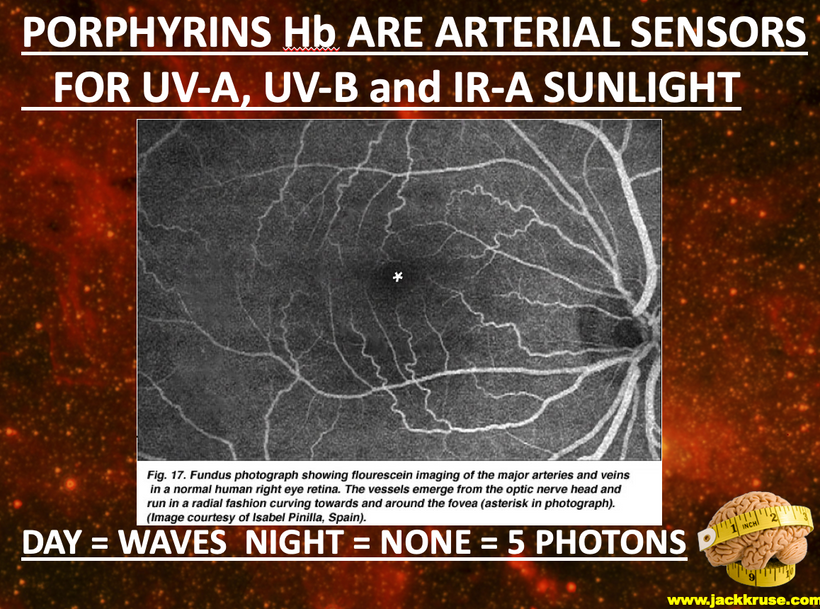
Melanin granules in mammals are strategically placed in cells being placed mainly in the perinuclear space (perikaryon), and completely surround the cell nucleus; which fully explains the operation of this protein. The RPE concentrates its melansomes at its apical end where the rod outer segment is engulfed by the RPE cell. This area doesn’t have the mitochondrial capacity that the basal end does that abuts Bruch’s membrane and the chriocapillaris of the choroid. This border mimic what we see in the gut.
Melanin can augment mitochondrial energy production in mammals. This energy source was critical in changing the body plans in mammals over the last 65 million years. This tells you melanin renovation is a critical piece of all mammal biology. This largely has been ignored by centralized science.
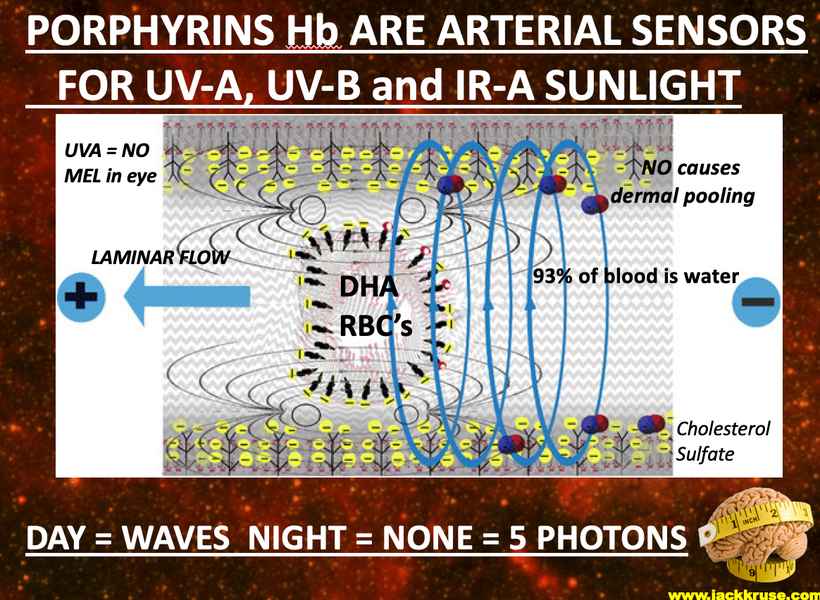
The perinuclear space and its melanosomes are surrounded by the rough endoplasmic reticulum (RER); which allows this organelle to capture molecules of hydrogen as they emanate from melanin sheets via charge separation. As long as there is a light source inside mammals water is continuously split into H+ oxygen and a massive pile of electrons that can be powered up and used in semiconductive circuits. They can be used to form another source of endogenous energy to meet the energy requirements of the cell. It is not solely the job of ATP as centralized science believes. This would have made Gilbert Ling very happy. This explained why Mitchell’s chemiosmosis model never could account for the ATP deficit a cell would run if Mitchell was right.
Why does this make sense? The RER in mammals doesn’t have mitochondria or ATP inside of it either. Many have forgotten this. Mitochondria touch the RER but they are not inside of it. Why is this odd? The RER in mammals is a semiconductor factory. In general, its function is to produce proteins for the rest of the cell to function. The rough endoplasmic reticulum has on it ribosomes, which are small, round organelles whose function is to make those proteins. It makes no sense to have no energy organelle in your semiconductor fabrication plant, does it?
Does nature make mistakes?
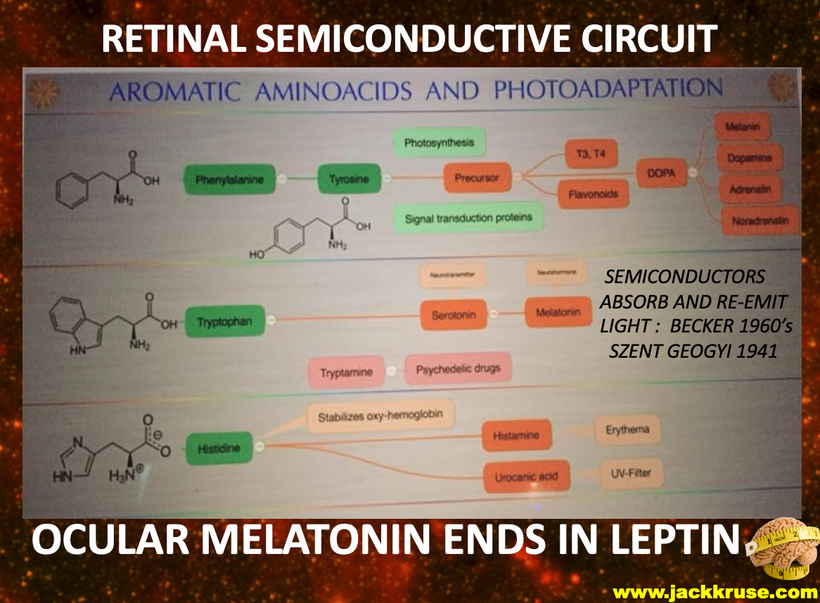
It makes sense when you realize cells are creating endogenous light to translate the POMC gene inside of their cells to make melanin sheets next to the semiconductive fabrication plant. This is the arrangement of the rods to RPE in the eye. The creation of the peptide bond is one of the most costly things in energy to make. Melanin had to be charge separating a ton of water to make a ton of H+ to create a lot of chemical energy. Ling’s stoichiometry told me to look for an energy-producing molecule other than ATP to satisfy Ling’s shortfall. Leptin was the accountant and it turned out POMC was the Source that filled the energy deficit in the eye.
HUMANS WERE NEVER MEANT TO EAT THIS MUCH
IR-A light increases energy production within the mitochondria. In fact, Infrared light from the Sun makes ATP within the cell through CCO (Cytochrome C Oxidase) without ANY FOOD ELECTRONS.
Fact: 42% of sunlight is composed of IR-A light to spin the ATPase.
Also Fact: a 70kg man has to make 85kg of ATP per day. This sounds nuts!
His body must produce his entire mass worth +30lbs of ATP every single day. The same is true of your body in the same proportions.
1/3rd of these excited electrons for ATP come from food.
2/3rds come from sunlight IR-A to spin the ATPase and the rest from melanin induced by UV light to create H+ to spin the ATPase and electrons to add to ECT.
Nature also put the Vitamin D receptor on the inner mitochondrial membrane to slow electron flow and get help from UV-A light to reduce ATP production via Nitric oxide creation…….
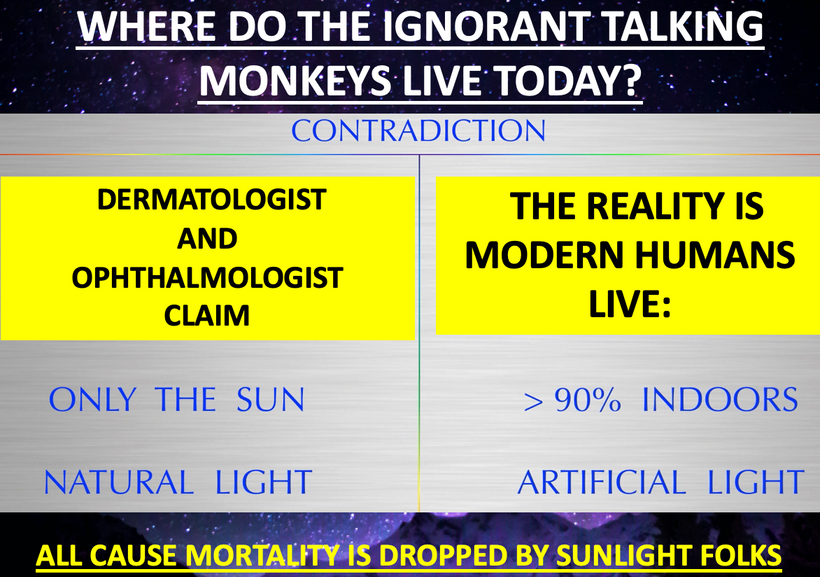
Or at least…they’re supposed to be in the sun………..
But how many of you are reading this while being buck naked outside in the direct sunlight?
I’ll guess 0.
Remind me again why it’s all about food…….?
You are a repository for sunlight. In the absence of star fuel, we have to eat copious amounts of food to maintain energy levels to live.
The more sunlight you get when you’re grounded to Earth the less food you need.
Go get that sunlight, because let’s be honest, we’re basically houseplants with complicated emotions.
This meme was created by Nick Stumphauzer and inspired by my work

This ability must be associated with a specific molecule capable of breaking symmetry. Melanin is a symmetry breaker too. This meant Noether’s theorem had to be in play, in my mind. H20 can “unfold” or ‘charge separate’ into H+ and -OH with the addition of infrared heat from a cell or the sun or when it lies adjacent to hydrophilic substances. Collagen and melanin are both hydrophilic.

I kept my focus on where hydrogen was and how it moved in the boxcars of biochemical pathways and realized the ionized form H+ would be affected by another universal law of nature = Noether’s thereom. Einstein’s relativity required Noether’s theorem to be accepted by physics long ago. Why?
So how does Einstein’s relativity directly tie to this short narrative on hydrogen?
Einstein’s relativity theory allows for space and time-bending (Noether’s theorem). It also bends the mind of many people who look deep enough to see how far-reaching this idea really goes in cells. It turns out relativity has a major effect on the elements of the periodic table. When electrons slow down, this actually increases their small mass because of the mass equivalence equation. For those of you who don’t know magnetism from the adjacent mitochondria acts to slow electrons down as a nuclear effect. I began to see a new perspective that allowed me to see another world I’d been missing in biochemistry textbooks.
Here we see a thermodynamic problem that must be solved. This small change causes the innermost electrons to get closer to the nucleus than usual. The longer-range effect of this shields the outermost electrons from the pull of the nucleus. This causes the outer shells of electrons to expand outward into space. Here again, when this happens, electrons’ energies decrease, and because of the E-mc^2 law, mass must increase. This explained obesity to me. As thing got larger we lost energy. I immediately thought of hydrogen and realized because it had double the atomic mass it would require more energy to move it in the boxcars of biochemistry.
FOOD IS NOT WHAT YOU THINK IT IS. THE LAWS OF THE UNIVERSE SAY IT’S NOT UNCLE JACK
This idea based in the theory of relativity of elements raised new questions in my head. For example, what happens to hydrogen when a single neutron is added to its sole proton and electron? This is what happens in deuterium. Does something unique occur atomically that I am missing? If an electron slowing down invoked relativity, and it made that big a deal, what the hell was the effect of adding a neutron to H+? In the subatomic world, a neutron is a giant compared to an electron. That question opened a new door in my mind. A door most of you won’t believe until you see it laid out.
A single neutron addition explained to me the food was not the key issue for health or longevity in mammals. Because of relativity, I realized an axiom in medicine that food is medicine is false. Why? Food can be medicine in certain environments and deadly in others because of Einstein’s relativity. It sounded nuts to me when I first thought about it, but I remember Feynman saying Nature is absurd in how she operates. So I held onto the idea to give it due diligence.
So I held this paradox in my head and thought it through.
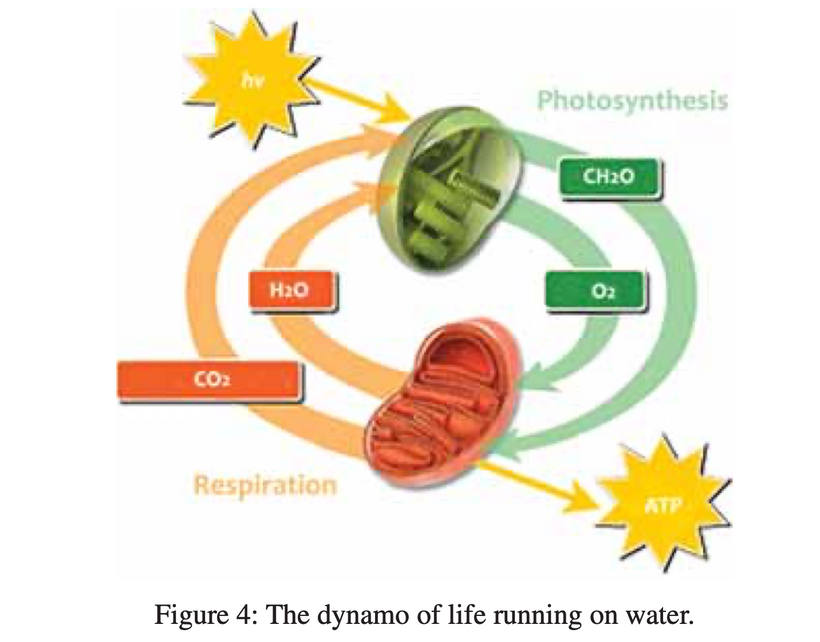
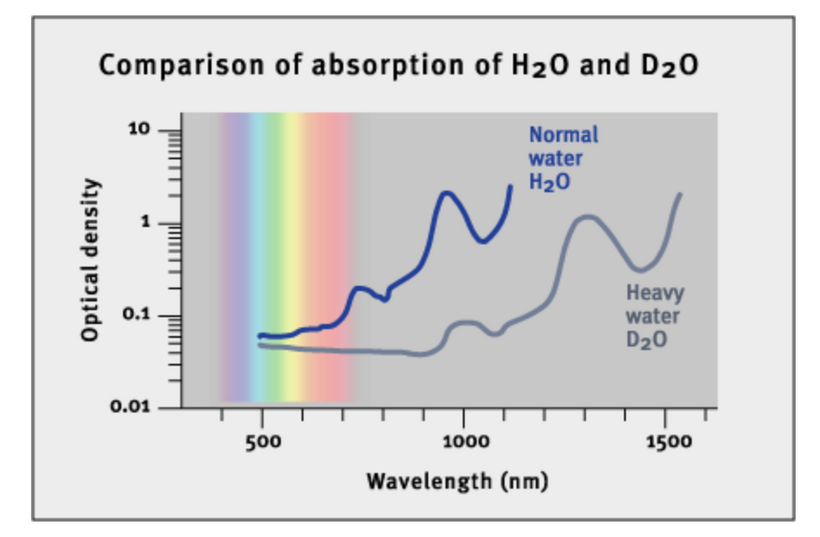
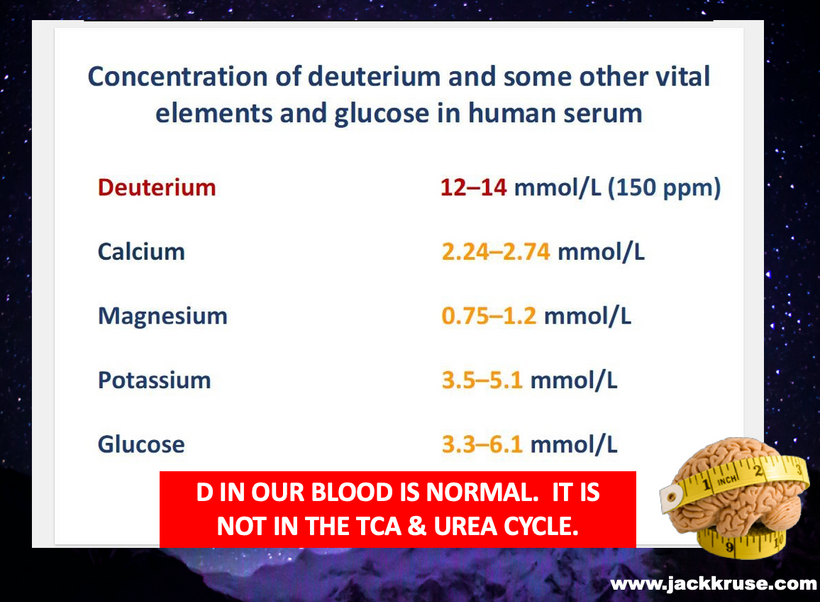
I thought about wild animals in Nature and the fact the food web is built 100% by photosynthesis using all forms of water on Earth at every latitude. Then I thought about the water a mitochondria was making around melanin, and what melanin was doing to this water. It was creating massive amounts of hydrogen but no deuterium because of the proton channel size in the ATPase. I knew something did not add up. Photosynthesis allows deuterium to be in foods, so this meant animals had to have all these fancy enzymes to isolate deuterium from the mitochondrial matrix. In fact, at this moment, I realized this is how ruminant mammals could tell the seasons and know when to migrate. Their brains weren’t developed enough to allow for this behavior so how did they do it?
Since wild animals cannot think to know when to migrate to seek new food, and they don’t have the ability to read a clock, I knew some other mechanism of telling time was operational. I realized they gained the ability to tell time from the amount of deuterium in their diets. Deuterium weight double H+ and it also had a radically different nuclear spin. Protons spin is also sensed by the mitochondria because all the channels in the mitochondrial matrix & ATPase are quantum nanomachines that are built exclusively for the atomic radius of H+ and not for the larger atom of deuterium = which is H+ plus one neutron.
This small change in mass in the animal enterocyte also invoked Einstein’s relativity if the electron did in atoms. Einstein’s relativity theory is why gold is yellow. In fact, this is the same thing used in an iPhone’s GPS system. The level of deuterium in the matrix of wild animals informs animals of this small change & therefore also simulates the season inside the matrix of the mitochondria. When I was a little boy, I went on a tour in the museum of natural history and I learned that all mammals membranes change their lipid rafts seasonally by altering their cholesterol levels. This is how their coats changed as light and temperature levels changed. Who knew that lesson as a kid would solve a mystery.
The amount of deuterium entering the matrix via UCP-2 alters the metabolic rate of the spin cycle in the TCA and urea cycles of mammalian mitochondria. These changes also changed the melanin coats on their surfaces.
This entire system is 100% programmed by the power density of sunlight in that season and this is how the ZIP CODE of seasons could be coded for the inside of all mammals. The more deuterium that is present, the more swelling would show up in the colony of mitochondria of their guts. This information could be sent to the brain via the peripheral nervous system and the autonomic system to adjust behavior and feeding. It could morphologically change its melanin coats externally just as a cephalopod or chameleon does because a TINY change in mass = a change in energy and when energy varies color and shapes have to vary too.
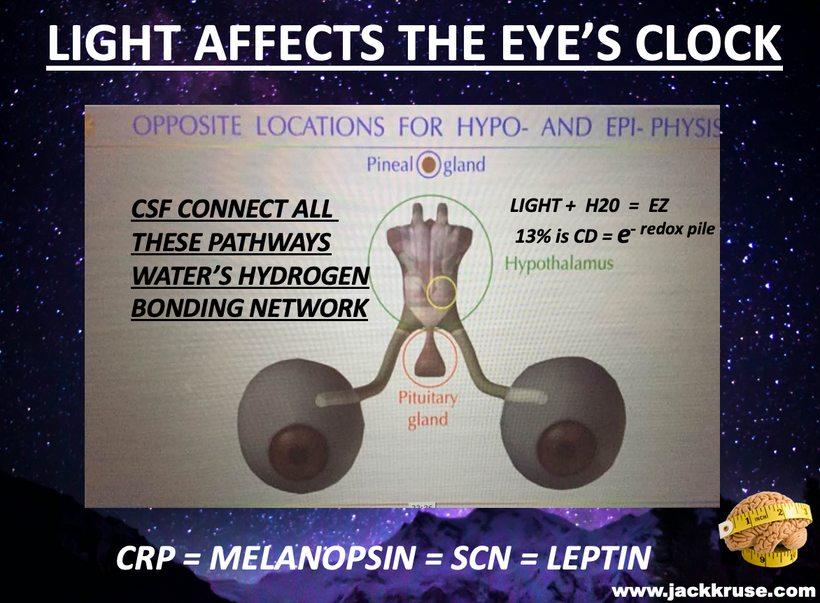
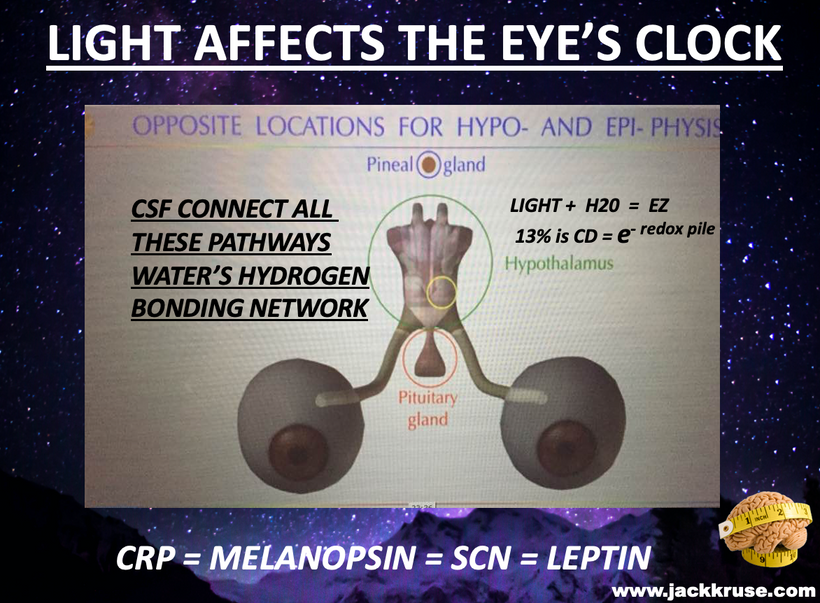
This shows you why thermodynamics and size and shape changes within the colony of mitochondria in tissues can be sensed macroscopically by our sensory systems in our thalamus which is filled with CSF made mostly from water. As you heard in the podcast linked above, the thalamus is the end target of the POMC neurons in the eye’s semiconductive pathway where light enters the system of timing. All the pieces fit in this quantum design. This alters the tensegrity of the mitochondrial system and the cell and changes water made in the mitochondria which surrounds every semiconductive protein in life. This changes the VUV-IR-A light a cell can transform.
I realized at the foot of David in Florence we’re being instructed by waves. We’re sentient beings made from atoms hiding behind a facade of box car biochemicals designed to hide the “magics” of the cosmos from our reason so we act/do/follow what the recipe requires. This allows the cosmic wand, the Source, to continue to direct our syncytium of atoms across space-time. From above and from below, the heavens instruct our cells how to properly behave to conduct the tissues in our bodies as the instruments which play the melodies capable of soothing our souls.
HOW DOES THE MAMMALIAN BRAIN LINK TO EARTH TO TELL US ABOUT OUR GROCERY LIST?
The mammalian thalamus also has normal resonant frequencies it absorbs and emits. It creates the 7.83 Hz alpha wave on EEGs which links all mammals to the heartbeat of the Earth. This thalamic frequency is tuned by molecular resonance to the Earth’s ionosphere as it revolves around the sun. The ionosphere resonant frequency varies seasonally and mammals can sense this too deep inside their heads. This allows them to alter their coats and semiconductors in cells to a changing light environment. Think of this system of signaling as the electric and magnetic GPS compass to monitor the position of the Earth as it revolves around the sun and it is communicating that quantum data to your thalamus. Anything in the thalamus linked to the thalamus in mammals also happens to be loaded with the POMC gene. This is the gene that codes for MELANIN. These electromagnetic signals are essentially informing POMC and alpha, beta, and gamma MSH to control feeding and link it with the photosynthetic food growth cycles. All the pieces fit perfectly. Now, many of you will now understand why Uncle Jack thinks most people do not understand food as they should.

DO YOU REALLY UNDERSTAND WHAT I JUST SAID ABOUT FOOD?
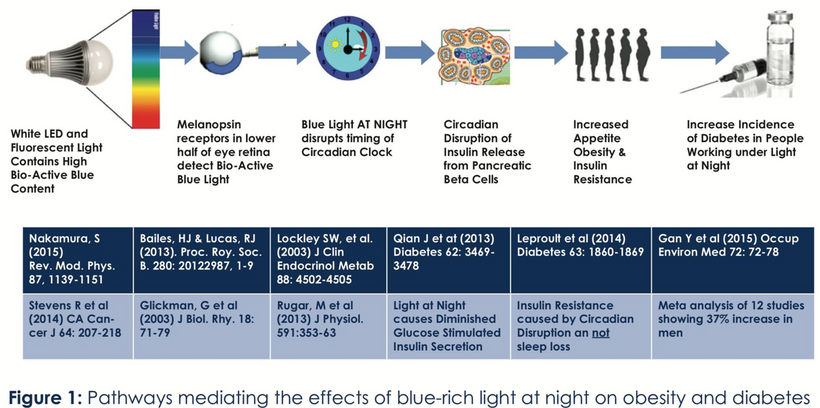
It means a banana eaten in Boston on December 31st is a MITOCHONDRIAL TOXIN, not a medicine as your doctor or bro scientist tells you. This is especially true if you are getting 12/31 sunlight at the 44th latitude via your skin and eyes. This creates a ton of inflammation in your colony of mitochondria. I said this as the keynote speaker at the first annual Paleo Fx conference and was mocked by their leaders.
When you eat out of seasons or in the wrong light for the food in question, you are making a MASSIVE quantum mechanical error for your mitochondria and central retinal pathways. Why? George Mourou & Donna Strickland got a Nobel for their development of Chirped Pulse Amplification (CPA) at the University of Rochester. In his speech, he referred to his “passion for extreme light.” What else did Mourou say in his acceptance of the Nobel Prize speech?
Read it.
Mourou said, “Take the nucleus of an atom. It is made up of protons and neutrons. If we add or take away a neutron, it changes absolutely everything. It is no longer the same atom, and its properties will completely change. The lifespan of nuclear waste is fundamentally changed, and we could cut this from a million years to 30 minutes!
Hard STOP for the disbelievers.
What did Uncle Jack say 4 years after Paleo Fx in Austin, in 2016 when I was in Vermont on this very topic? Could deuterium turn healthy food into a toxin?
I showed a picture slide and asked the room why no one stuck at the biochemical level of understanding of food ever ask themselves this question. Remember they were all Weston A. Price folks………….nobody said a word.

The answer was to isolate deuterium from H+. The lesson of the periodic table was a huge piece to this puzzle.
I came back two years later and showed how a cells in our skin and circulatory system can make VUV light. I showed the mechanism used in the blood and skin. I sent this Tweet message out and link to the Mourou Nobel Prize talk and asked this question what happens to foodstuffs when you add a neutron to H+? Is the food the same healthy food or does it become a poison? I mentioned that processed keto food that all the guru sold via MLM schemes sets off the RF detectors at airports too. I asked them why? I asked them does it matter?
Radio silence.
I asked a simple question, “If one takes the nucleus of an atom in food, it is also made up of protons and neutrons like nuclear waste. If we add or take away a neutron, does it change the food?
ANSWER: it changes absolutely everything we think we know about food and its properties because the atom is different. What does that mean if you are a mitochondria? Do they react to it like glyphosate?
Might we be blaming a lot of bad food ideas when the real toxin is the food just eaten out of season or shipped from another hemisphere where it is summer
After all, as Mourou said, it is no longer the same atom, and its properties will completely change, and a mitochondrion won’t be able to process it the same. This is what light does in photosynthesis between H+ and deuterium. Photosynthesis puts deuterium in certain places on the backbone of food carbons and enzymes subtract the deuterium away to shunt them from the matrix.
This is why biochemistry has all those enzymatic steps in the slide above people. Everyone opens a biochemistry book but no one thinks to ask the question that is obvious. Why is Nature doing all these steps? Does she make errors? Does she add enzyme steps to drive medical students crazy during their first year of training?

The matrix pays attention to this change EVEN when you do not at WHOLE FOODS on 12/31. What happens? The lifespan of disease creation is fundamentally changed in human mammals from far off when you are old, to diseases rapidly showing up in children and teens. This is why I can see diseases of aging in 20-year-olds in my clinic.” HEALTHY foods can be toxins when you have this perspective.

Now let’s review again what Mourou said in his acceptance of the Nobel Prize speech on the same topic. He said high-intensity endogenous light made in a lab could cut the lifespan of nuclear waste because it is fundamentally changed, and we could cut this from a million years to 30 minutes.
Why do Mourou and Kruse sound familiar on this topic about neutron additions? Because they understand the physics behind the actions in Nature. Most do not because they suck at observing what she is doing at the smallest levels of science. Therefore, they call us crazy motherfuckers when it’s the ignorance of centralized systems of science who are out of line with their outdated thinking.
So when you hear me say something about food ever again…………remember this blog passage. Tape it to your forehead and maybe tattoo it on a body part. No one understands food like a mitochondriac. NO ONE.
The implications of my podcast with these two men goes way deeper than most of you can fathom. I’m still warming up.

Nature’s food lesson for us all: Just because something seems logical about food, doesn’t mean it’s wise. Something can make sense in and of itself, but when tested against reality yield an undesirable or inefficient outcome. Being seduced by impractical logic is antithetical to wisdom, you just feel smart despite being wrong. Nature is absurd in how she uses isotopic variations in food in mammals.
BACK TO THE PERIODIC TABLE SCULPTING MAMMAL STORY
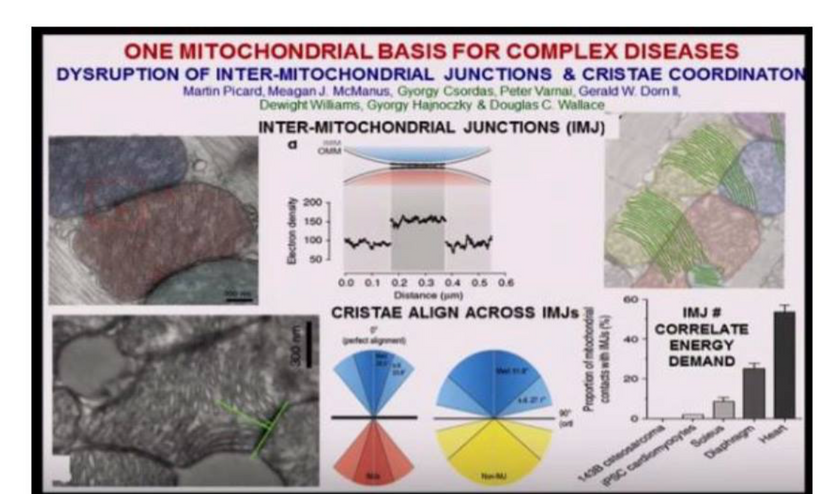
Post-KT event, the absence of UV light allowed melanin to move from the exteriors to the interiors where melanin adaptation and renovation were maximized. The results were seen in massive alterations of mammalian body plans within ten million years. These changes required energy. Where did it come from? Well, the sun returned and now melanin was inside and outside most mammals. 50% of the energy-consuming processes of the normoxic cell in these animals can be accounted for by ion pumping. This is even true in a neuron. The transformation of light energy to chemical energy by melanin inside of the body’s tissues serves this need for energy. It decreased the need for membrane ion leakage to generate energy. This ability contributed immediately to a major energy saving, This allowed for organ expansion and rearrangement like encephalization seen in mammals post-KT event.

Theoretically, the power to the inside of the cell depends partially on ATP as Ling always believed. Melanin filled the rest of the thermodynamic gap Ling saw in his calculations of ATP stoichiometry. Internalized melanin gave mammals the ability to transform all light energies into chemical energy by means of water dissociation into hydrogen, oxygen, and 4 electrons. H+ is used to spin the ATPase Fo head to create ATP and a magnetic field. Mammals create massive amounts of H+ from melanin’s ability. This helps explain why Ling was furious with Peter Mitchell for decades. He had no idea about what melanin was really doing in a cell because I asked him about it. Energy production in mammalian cells relies on massive additions to the energy balance sheet because of melanin. When you look at Ling’s numbers for ATP stoichiometry, the math didn’t add up. I knew right away, why Ling was right.
TYING IT ALL TOGETHER
H+ and magnetism help explain why we use the atoms we do on the periodic table. The key ones that make VUV light are all paramagnetic. They are drawn to the mitochondria because that is where melanin is to make H+. Many people want to understand how magnetism fits into the 3 legged stool of life. Free radicals are all drawn to magnetic fields too because this is where mitochondria and melanin are in mammalian cells. These areas in cells are where the sun buries its magnetic flux in cells and connects with it wirelessly.

It uses H+ creation via melanin and matrix water to do it. Magnetism is the essential force that determines the form of plasma or ionized matter taken in an environment. The hydrogen regions around galaxies are also considered plasmas, despite their degree of ionization being small. The degree of ionization in interplanetary space varies between unionized states or can morph into fully ionized states in other regions of space. In space, however, even the weakly-ionized plasma in the hydrogen region reacts strongly to electromagnetic fields. Mitochondria at small scales have a massive electromagnetic field with respect to H+.
Magnetized plasma, such as that contained in the hydrogen region, is the dominating state in the universe as a whole. Our sun produces massive amounts of plasma it spits out into the solar system as the solar wind or a coronal mass ejection. The sun’s plasma is contained by the high electric and magnetic fields of the sun. So is the H+ in our mitochondria to create ATP. This makes your colony of mitochondria filled with H+ a wireless antenna for the sun’s photons. Our circulatory system connects us to the magnetic flux in the sun.
NOETHER’S THEOREM EXPLAINED FULLY IS A FUNCTION OF POMC LOCATION
Melanin captures that energy from the sun buried in our blood. As the energy is captured, Noether’s theorem is used to bend space-time in cells to tell matter how to act in cells. This is covered by the field of general relativity (Einstein), which treats gravity as a bending of space caused by mass and energy.
Einstein’s theory seemed to have a serious problem at its core: Energy caused the bending of space, but gravity itself was energy in his model. Thus, it would seem that the energy of bending space made yet more energy. Presumably, this would bend space more, resulting in more energy transformation. It seemed like the theory could cascade in this manner to the point that energy would grow forever. And because this didn’t happen, there needed to be a solution to the problem. Emmy Noether’s solution to the problem is now called Noether’s theorem. Her theorem revolutionized physics, but her theorem has yet to hit biology because biology still doesn’t realize that all biochemistry is quantized by light via non-linear optics in cells.
Light, captured by melanin, tells cells how space-time bends at the electronic level inside of us. ——-> POMC CHANGES SPACE TIME IN YOU!
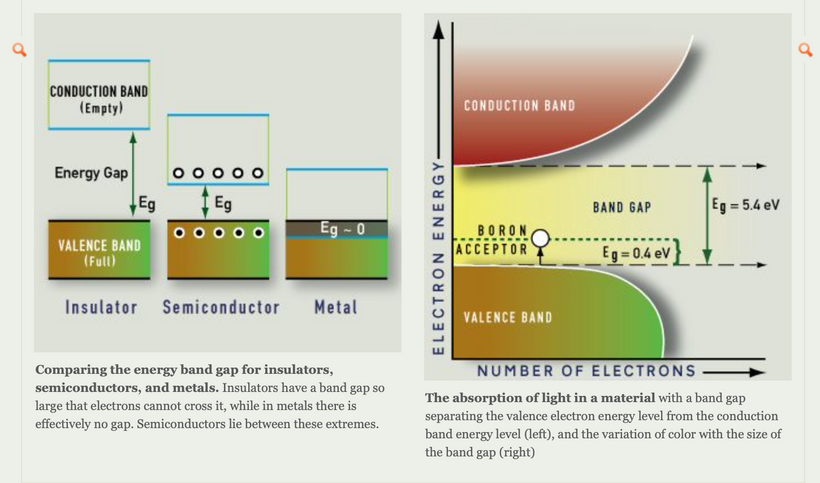
This is how the wide band gapped semiconductors are organized quantum mechanically below your ability to sense it from atoms and by relativity to make light waves stronger than the sun provides inside of us. To decipher the electric and magnetic codes you need wide-band gapped semiconductors to get Nature’s recipes to run your cells. This light is what keeps cells operating far from equilibrium to satisfy the second law of thermodynamics.
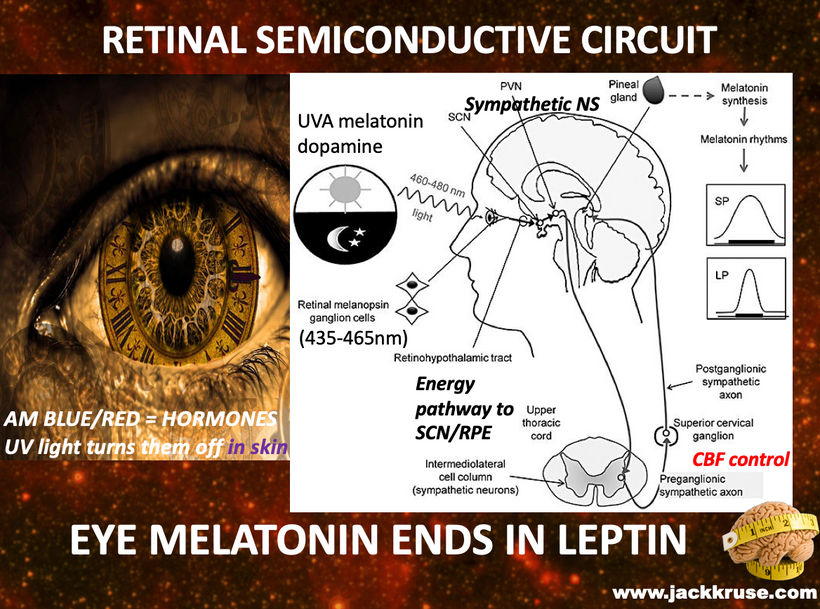
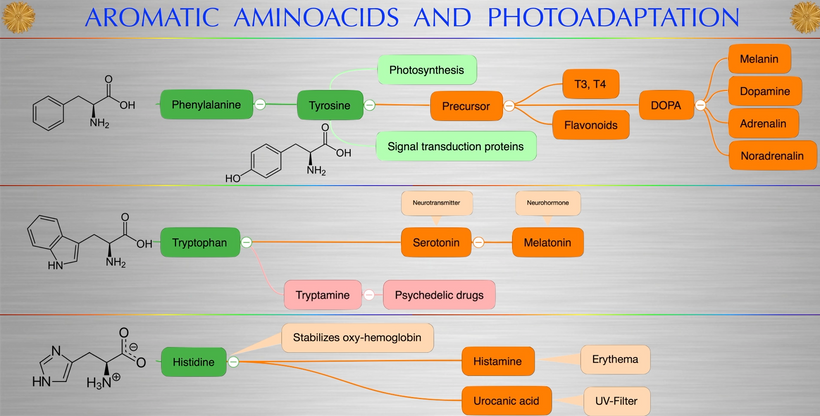
This arrangement is really interesting in the RPE of the eye and the choriocapillaris. In mammals, moderate hypoxia or pseudohypoxia (14%) significantly increases the extracellular concentration of dopamine, prior to retinal depolarization in retinal ganglion cells. This hypoxia is critical in degrading melanin into DOPA to create dopamine in the eye to keep the globe round and not myopic while also helping regenerate photoreceptors with melatonin which is created in the mitochondrial matrix. Dopamine and melatonin are both assisted by DHA breakdown products in the eye in preserving photoreceptor regeneration. I believe DHA is critical in melanin renovation as well. Why?
Uncompensated oxidative stress is often an early event associated with retinal, neuronal or heart cell death, and the retinal pigment epithelium and retina are under continuous stress by nature’s design. One of my mentors in neurosurgery, Dr. Nicolas Bazan, found that elovanoids created from the photooxidation of DHA have unique structures and that they enhance the expression of pro-survival proteins in cells undergoing uncompensated oxidative stress by controlling autophagy.
Bazan and his team at LSU found that ELVs are made from 32 or 34 carbon-length fatty acid precursors produced naturally in human retinal pigment epithelial (RPE) cells. Most of the known lipid mediators or messengers are derived from 18, 20, or 22 carbon-length fatty acid precursors, including prostaglandins, leukotrienes, lipoxins, endocannabinoids, resolving, and docosanoids. Elovanoids have structures reminiscent of docosanoids but with different physicochemical properties and alternatively regulated biosynthetic pathways.
That elovanoids are longer than all known mediators may be the key to their potency. Dr. Bazan spoke to me this week and told me he just discovered two more ELVs that are 43 -46 carbons. He was very excited about their potential use in TBI cases. The longer elovanoids may be able to reach and bind for a longer period of time to receptors in cells necessary to induce cell survival. I have a sense these longer ELVs may protect the non-visual photopigments in the retina, brain, and carotid system. This will become important later in the series.
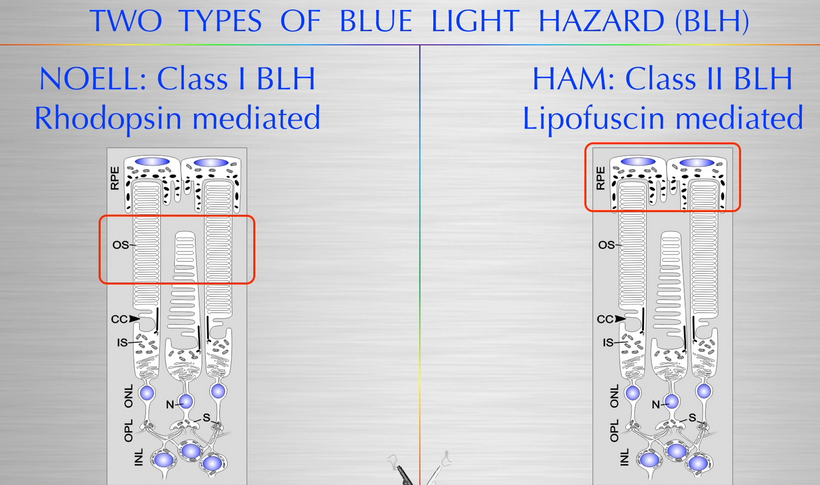
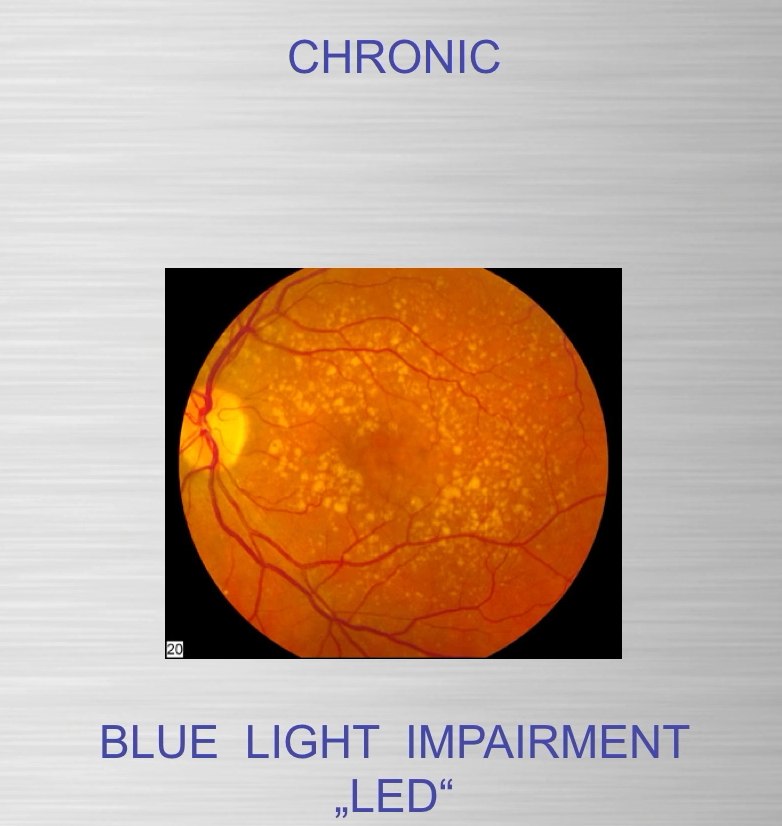
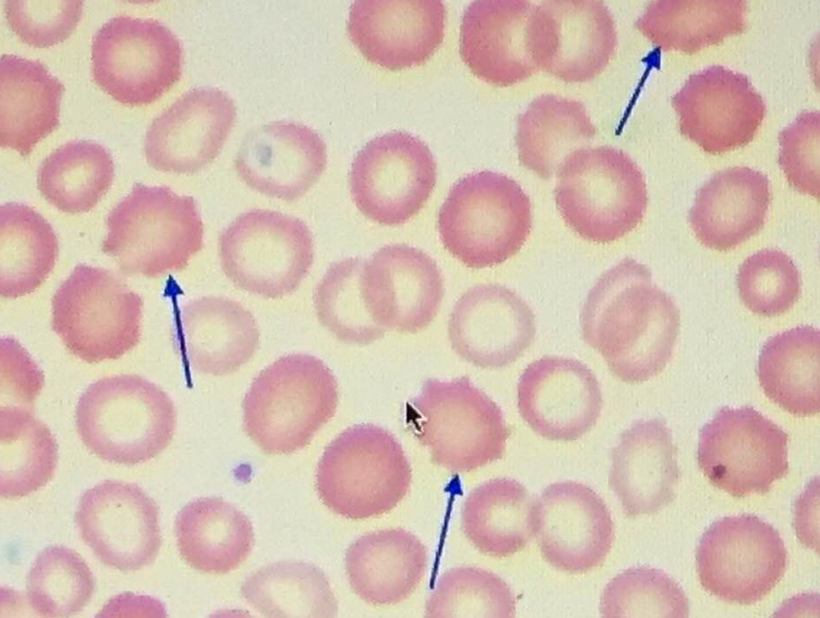
People forget the reactions on the top line of the slide below are bidirectional and hypoxia controls which way we go, right to left = normoxia, but left to right is done in hypoxic environment causing melanin to degrade. Hydrogen atoms force this change at melanin interfaces because they are atomic chameleons.
Not too hard to understand once you see it laid out in this blog.

SINCE WE CREATE LED LIGHT FROM VUV SEMICONDUCTORS…….WHO IS THE LIGHT SHOW FOR INSIDE OF US?
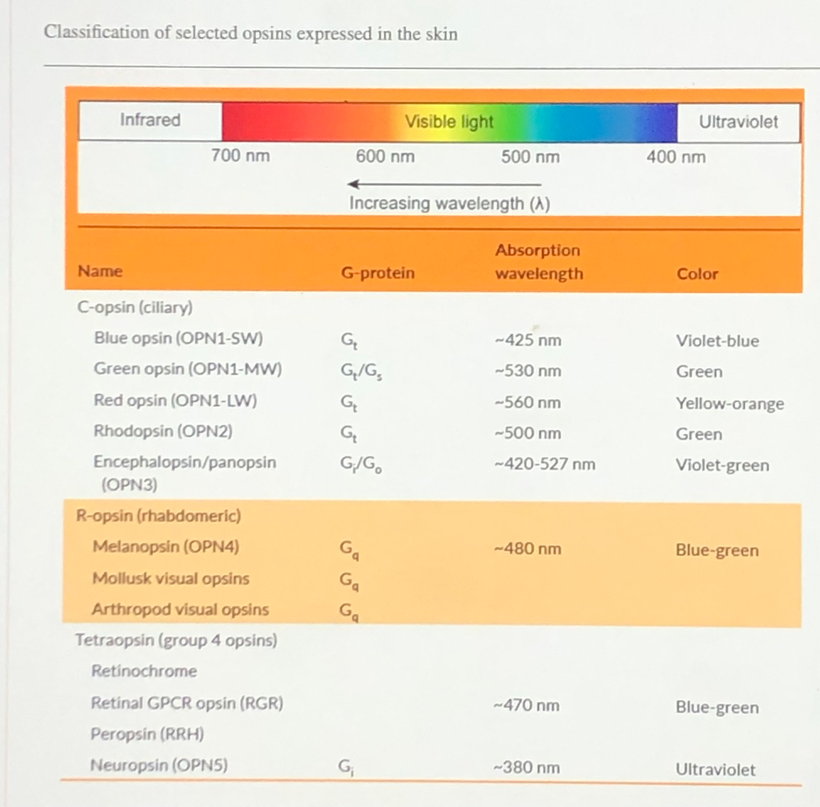
If you remember the dialogue between Dr. Huberman and myself on melanopsin he remarked boldly that no one in his PEER group could believe in the early 2000s that humans had rhabdomeric opsins from non-vertebrates in our body plans. Although the melanopsin system of mammals has received the most recent interest, the founding member of this new branch of the opsin gene family was in fact isolated from the photosensitive dermal melanophores of Xenopus laevis, a frog in 1998.

You guys could not see my face during the podcast but I had a real big smile on my face as he said it. Why? I thought everyone knew the story of where mammals came from. I learned it as a young boy in NYC museums. I was taught although there was no abrupt transition to ‘true mammals’, the general idea is that the tetrapods (vertebrates with four legs) divided into amphibians (that lay eggs in water) and amniotes (that lay eggs on land).
Amniotes then split into sauropsids (including dinosaurs) and synapsids (including mammal-like reptiles), which eventually led to mammals. Once the dinosaurs were gone, early mammals could stop living nocturnally and flourish in the many forms we find today. So for me, having frog melanopsin in the human brain made total sense It also told me we had to have deep chromosomal stability in the mammalian genome. It turns out my hunch was right (below).
The discovery in the early 2000s by David Berson’s group at Brown University of other cells in a mouse retina that responds to light and has nothing to do with a vision came as a shock to centralized science. Dr. Huberman confirmed this in Malibu. None of them thought non-visual photoreception was possible at this time.
Many mocked Suzanne Sommers when she reported in the 1990s that a penlight behind her knee affected her melatonin level and disrupted her sleep. Even stranger discoveries became commonplace in many laboratories demonstrating that these cells contained a new class of opsin proteins called melanopsins, never before seen in vertebrates (but similar to those of many invertebrates like fish). Non-mammalian vertebrates retain two quite separate melanopsin genes, while mammals have just one. Here is the screens for all the light show created by your interiors.
Every modern mammal, from a platypus to a blue whale, is descended from a common ancestor that lived about 210-180 million years ago if they are a mammal. We don’t know a great deal about this animal, but the organization of its genome has now been computationally reconstructed by an international team of researchers. The reconstruction shows that the mammal ancestor had 19 autosomal chromosomes, which control the inheritance of an organism’s characteristics outside of those controlled by sex-linked chromosomes, (these are paired in most cells, making 38 in total) plus two sex chromosomes.
KEY TAKE HOME: Mammalian chromosomes have been more stable than scientists predicted before the human genome project was complete. It turns out, Darwin was wrong.
The researchers found nine whole chromosomes, or chromosome fragments in the mammal ancestor whose order of genes is the same in modern birds’ (therapod dinosaurs) chromosomes. It seems it is very likely there was some crossing of genomes between birds and mammals at the KT event since these two animals made it through the last extinction event together. Their genomes show this connection. This only deepens my theories about melanin.
More proof these animals are linked to POMC biology: The researchers found in contrast, regions between these conserved blocks contained more repetitive sequences and were more prone to breakages, rearrangements, and sequence duplications. Ancestral genome reconstructions are critical to interpreting where and why selective environmental pressures vary across genomes. This study has now established a clear relationship between chromatin architecture, gene regulation, and linkage conservation.

It does something else centralized science doesn’t like. It provided me with hard data to say publically that the foundation for assessing the role of natural selection in chromosome evolution across the mammalian tree of life isn’t Darwinian.
It follows a quantum periodicity that links it to the periodic table of elements and the amplification of the use of paramagnetic atoms to build semiconductors in cells. Why? Chromosomes are filled with base pairs that are magnetized by UV light. That periodicity is in where hydrogen is and isn’t and what hydrogen’s atomic weight is in that zip code, and what its magnetic moment is while in that zip code. You’ll see why this idea is on solid footing soon enough.
In the study, they found that the rate of chromosome rearrangement differed between mammal lineages. For example, in the ruminant lineage (leading to modern cattle, sheep, and deer) there was an acceleration in the rearrangement of these chromosomes 65 million years ago, when an asteroid impact killed off the dinosaurs and led to the rise of mammals.
Centralized sciences call this a “remarkable finding” because their theories from Darwin wouldn’t have predicted it. I chuckled when I read the paper in late 2022. It shows definitively, that mammals have enjoyed evolutionary stability of the order and orientation of genes on chromosomes over an extended evolutionary timeframe of more than 320 million years. The doesn’t sound like natural selection as it was taught to me.
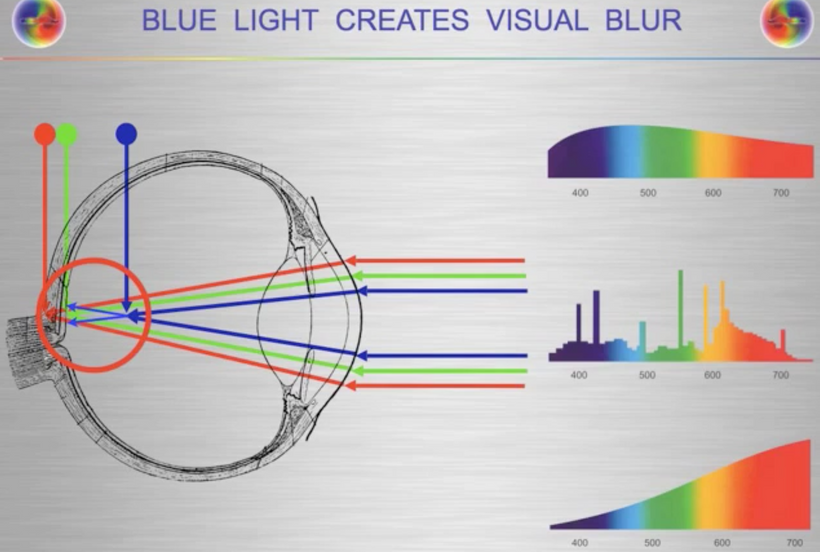
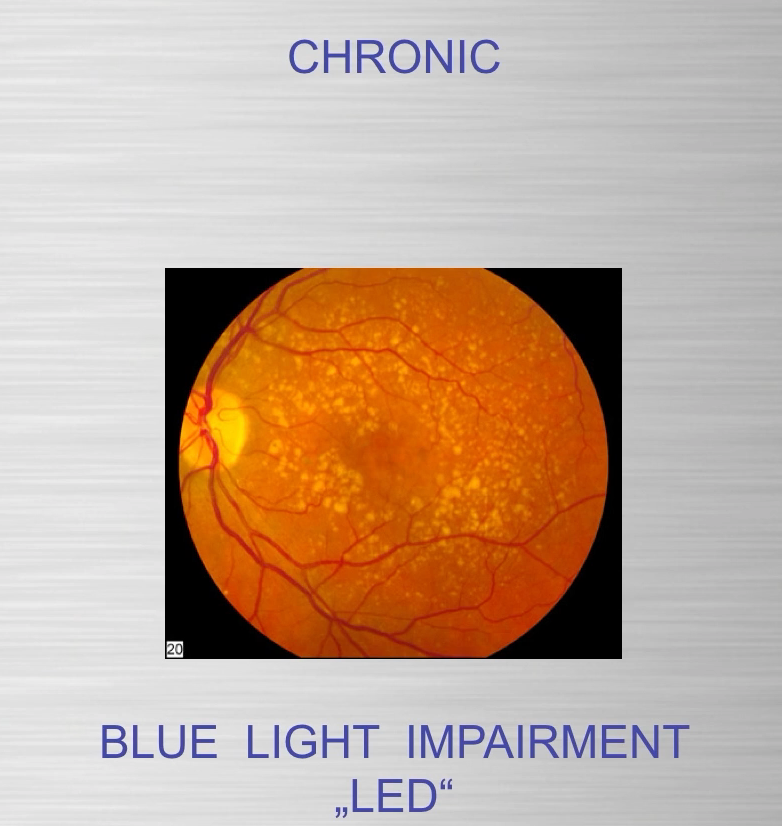
We now know that melanopsin is the most common opsin in the human brain even though blue light cannot penetrate the bone in our skull. It can only get in via the eye and has to pass the RPE of the retina before it gets deep into the substance of the brain.
SUMMARY
Centralized researchers forgot Nature’s lessons. Molecular oxygen (O2) is essential for vertebrate life. Strikingly, certain species are able to survive for periods lasting several months in anoxic conditions and are able to recover full function at the end of this time when O2 is restored.
The freshwater turtle Chrysemys picta is a well-studied example. It spends long periods during the winter in ice-covered ponds without access to the surface, often in water or mud with little or no O2. Mammals evolved 210 million years ago to live underground outside of the sun in a hypoxic environment. It seems melanin helped them navigate these environments.
This pseudohypoxia was linked to the NAD+ levels of their mitochondria and when oxygen was low and blue light dominated Earth’s surface and the dinosaurs were gone this allowed melanocytes to migrate inside of us. This brought melanin closer to our colonies of mitochondria where the real action began in energy transformation to create a human brain.
I’m closing in on the recipe of light I mentioned in the podcast. It turns out the beginning of Genesis never told us the shadows in life hold the light in our perception of reality. It might turn out that evolution theory and Genesis both contain half-truths.

CITES
My cerebral cortex