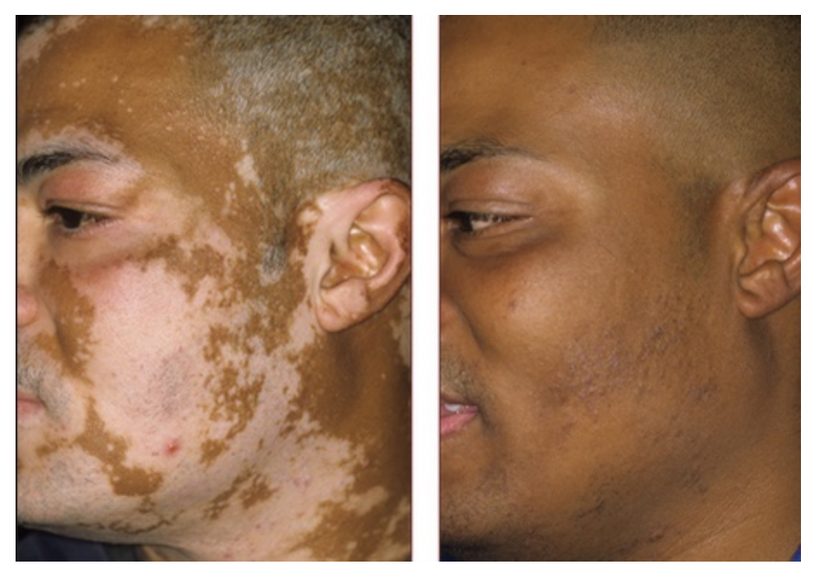
This blog might be the most important thing I will ever write for the public’s health. It is more powerful than any of the leptin blogs or the Cold Thermogensis monster blogs because it links them all together in one space.

This idea all began with a Eureka moment at the foot of a statue (above) in Florence close to 20 years ago, after reading a book that was a fable. What followed was a synthesis of many different branches of science to come up with something called the Leptin Rx seen below.
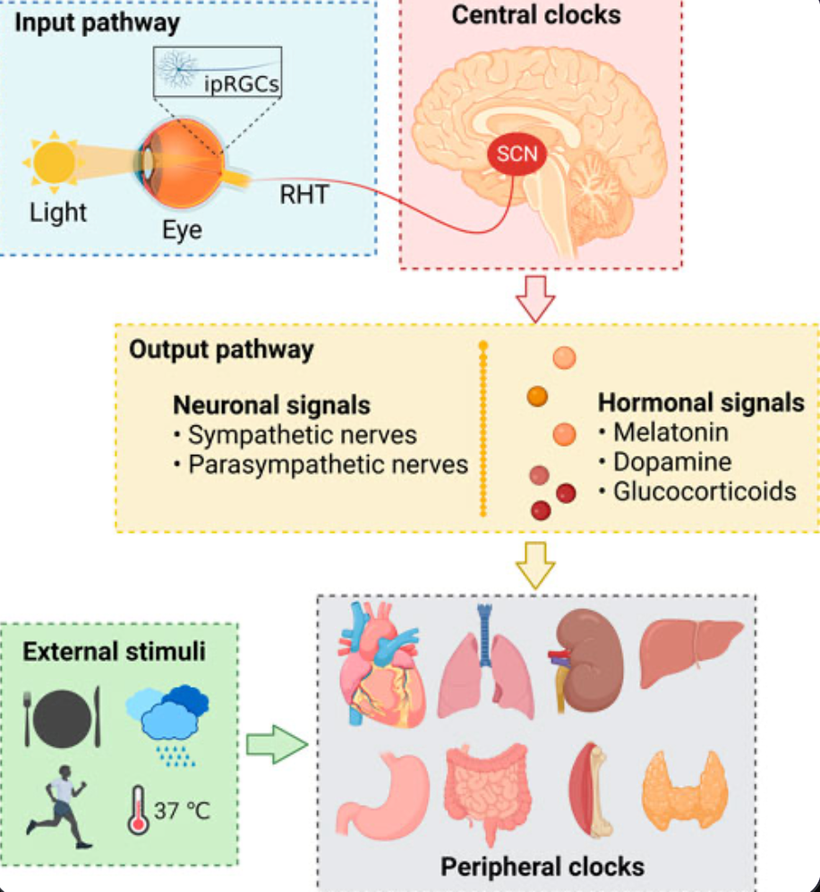
That picture above was a skeleton of an idea. The things I knew and the things I was taught about the SCN and circadian biology in neurosurgery had to be connected to the things I was learning about the eye, skin, and liver, like a QUILT. Then the picture above, evolved into the one below.

When I had put it all together I was ready to share it with the world…….So I did at a TED talk. The talk was good but immediately I was in trouble with three state medical boards, my professional guild, the hospital CEO, and then DoD. Below is an actual picture taken by my family as they sat below the cartoonist as he drew as I spoke.

This is what a blow up section of my part of the cartoon showed above. I submit to you everything on that picture below is what I have been teaching the world for the last two decades. You may not see it all in this photo but after ready today’s blog you should.
So what are the final pieces you need to put together to see it all for yourself?
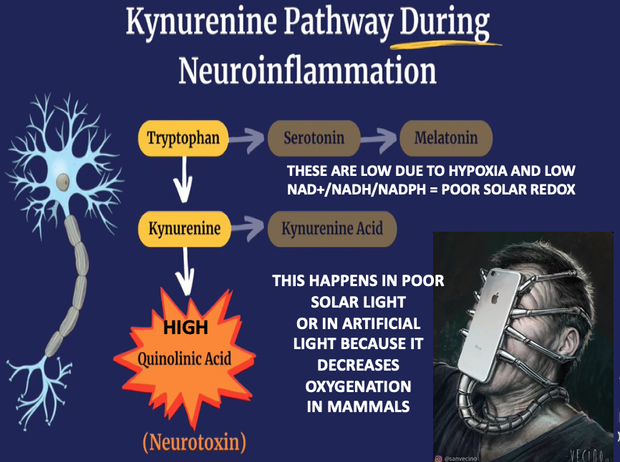
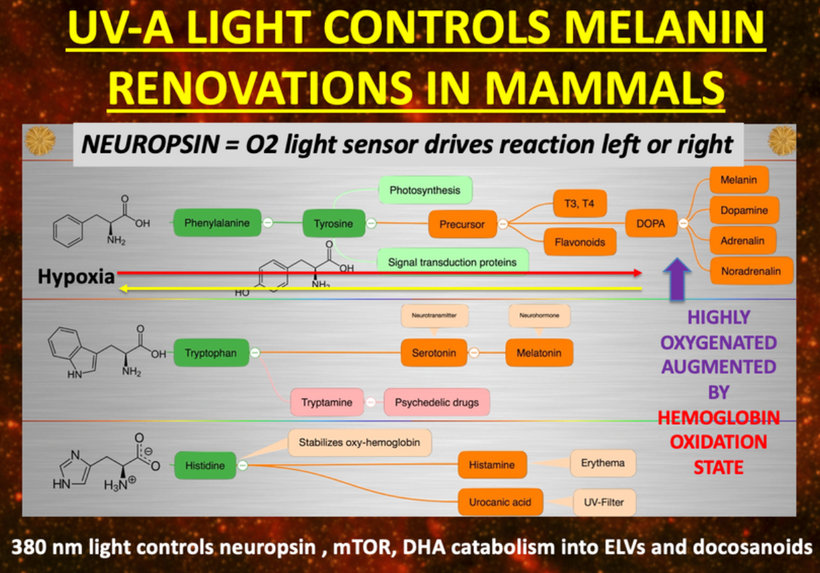
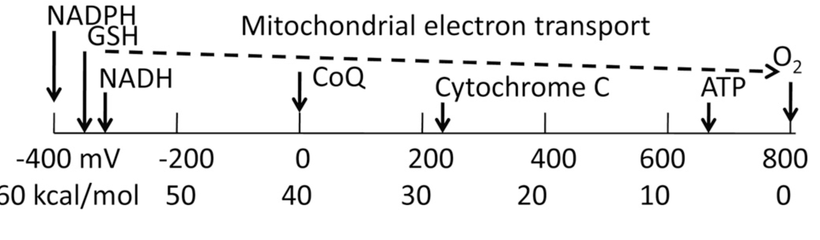
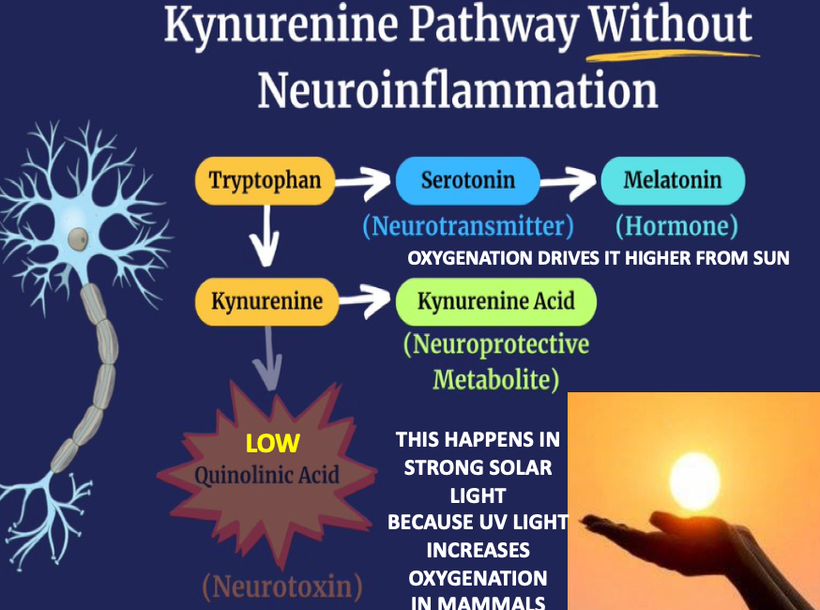
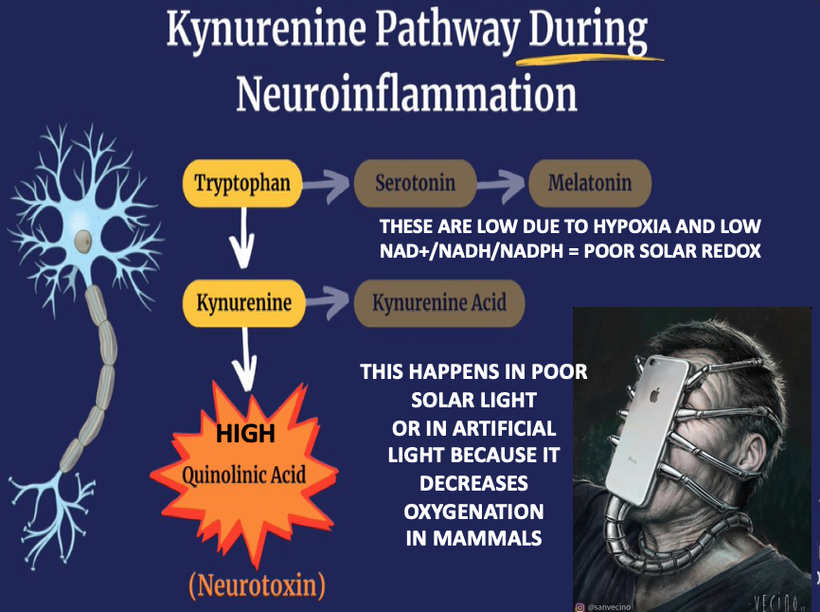
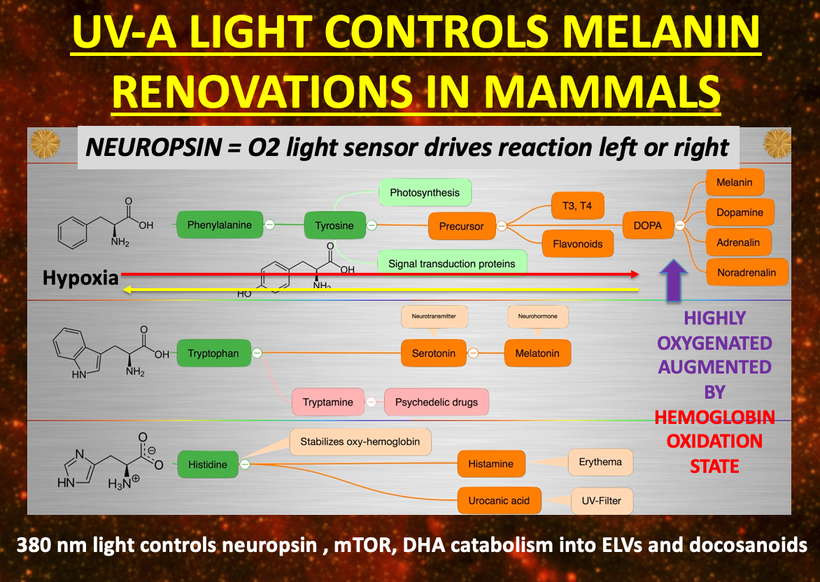
If you read my Patreon work on tryptophan’s role as a protein semiconductor and its seasonal role as a time crystal then understanding this post will be easier. Sunlight reduces inflammation by lowering the proton content in the cytosolic water and making sure protons stay inside the mitochondrial matrix. As a result negative charge density builds in the cytosolic regions of a cell. This high net negative charge is known as a high redox state. Persistent chronic inflammation in neuroectodermal derivative slows the production of serotonin, steering it instead toward self-destructive quinolinic acid production. Quinolinic acid comes from melanin degradation. Melanin can be made from UV light exposure of our eyes and skin via alpha MSH cleavage of POMC.

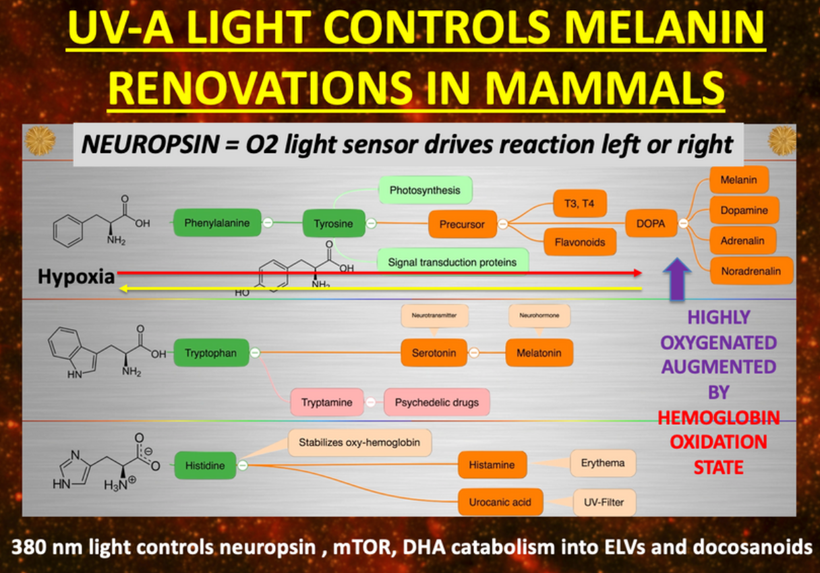
The breakdown products of melanin are thought to play a role in many psychiatric symptoms associated with chronic inflammation and infections of the brain’s frontal lobes. I think it plays a leading role in all chronic diseases. Below you see how melanin is made.

Below you see that melanin degrades…….but what does it degrade into? Every single compound it degrades into is highly inflammatory and leads to hypoxia in cells. I told you that in this series as the slide below shows.
Without sunlight, melanin is eventually degraded into quinolinic acid. This compound destroys charge density in a cell causing dielectric collapse in many systems like mitochondrial water and DHA loss. It mimics the effect of fluoride deposition in a cell.
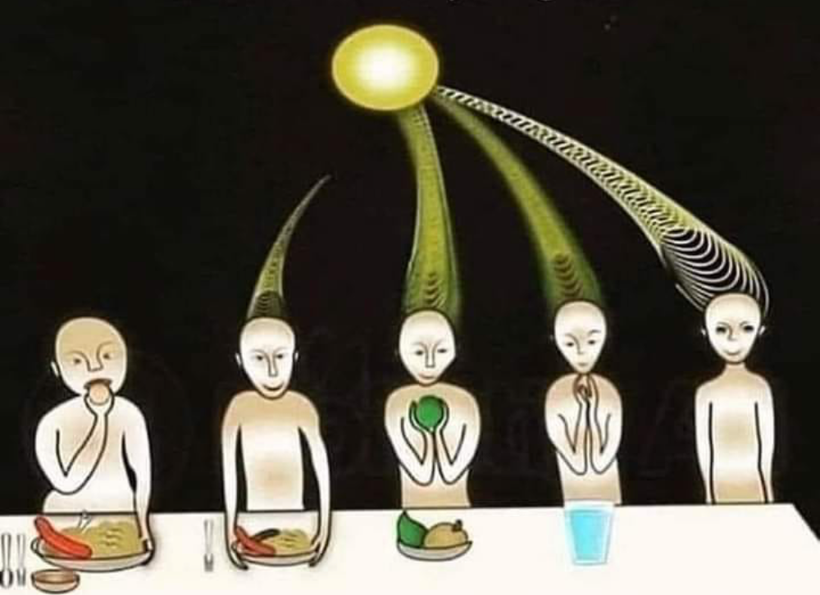
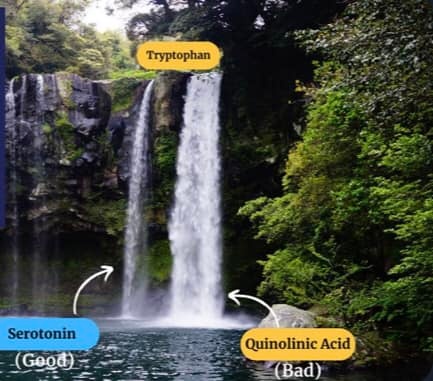
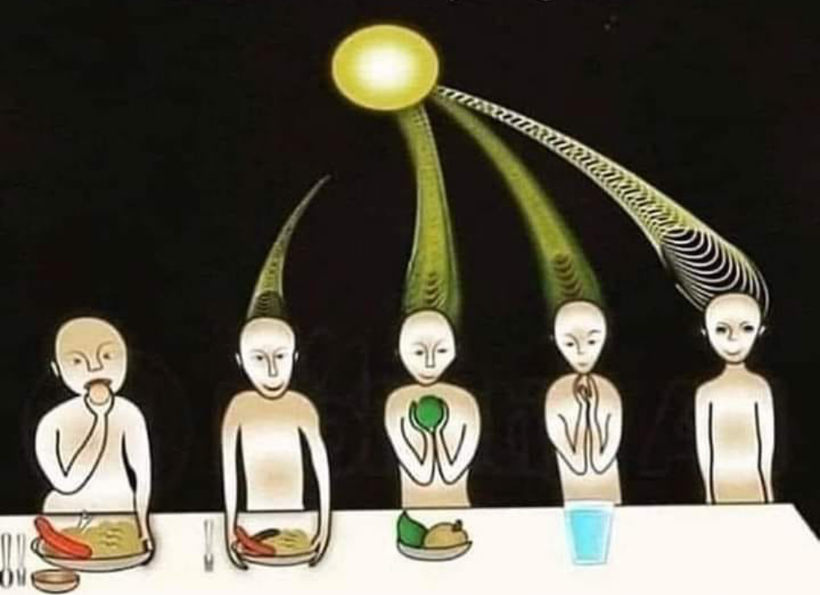
Sunlight exposure sets the metabolic efficiency of how the pathway operates. The image below of a water fall accurately represents the relative efficiency of the kynurenine pathway when solar redox is optimized.
I told you things made from Tryptophan were special because they acted like a time crystal. They actually pulse with a peridicity to the photons in the sun. They are a time crystal. I even told you this had implications for things made from tryptophan. Serotonin, melatonin, and NAD+/NADH/NADPH are three key semiconductive proteins in this story on melanin. Carefully look sequentially at the next few slides. Do you see any trend that links all of them together?

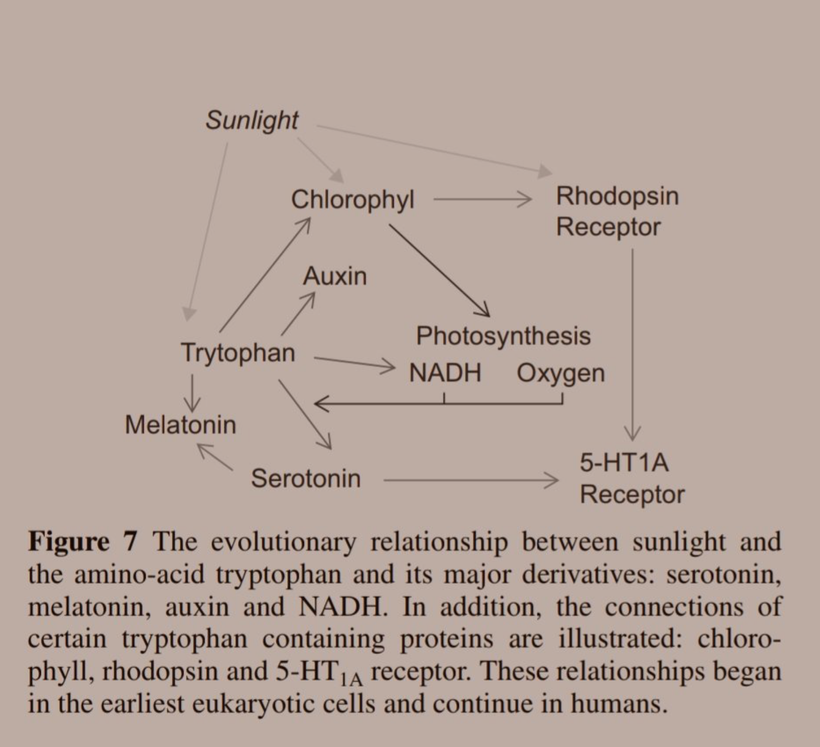


If you do not see it, I likely cannot help you. But if you do see that light, light from the sun affects every single key mechanism that controls biochemistry flow in us then my work is resonating in your brain.
Did you know that antipsychotic drugs, like chlorpromazine stimulate melanin in the brain? It seems that psychiatrists forgot this fact I learned in medical school over 40 years ago. Centralized psychiatry and their partners in Big Pharma wants you to believe, the mechanism of action of these drugs has not yet been definitively determined. See the papers from (Richtand et al., 2007) and their efficacy varies from individual to individual (Remington and Kapur, 2010) in a manner that is not always related to specific blood level values (Buchanan et al., 2010, Zhang and Malhotra, 2011). They used the PEER review literature to plant the following seed: Why these drugs works is a mystery. Why? Because if you did understand it, you’d understand that melanin is created from sunlight and that is all one needs to reverse most diseases. That is why the DoD wanted to meet with me in the early 2000’s and it is why Big Pharma had my TED talk banned. Look at the blue highlighted area below.
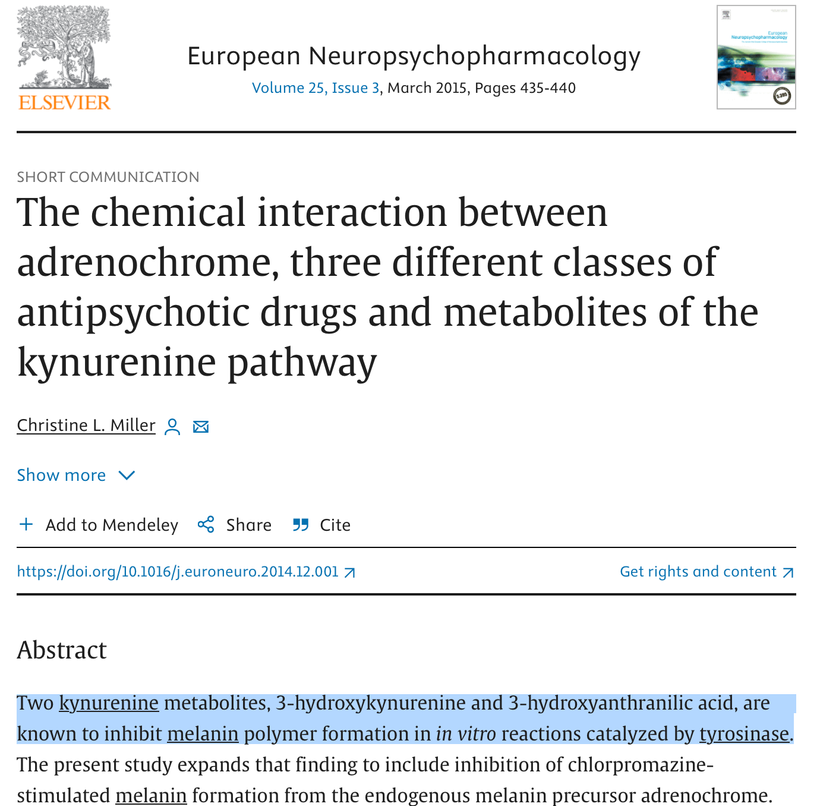
Antipsychotics work because they help put melanin back in a mentally ill brain by restoring some VUV light creation in your mitochondria during metabolic respiration. That is why I showed this slide in Vermont in 2018 below. Before you ask, no you do not want to use these drugs to renovate endogenous melanin because the side effect profile is too dangerous in low redox mammals. This is why these drugs have a low margin of safety. Sunlight however is just about risk free and this is why the DoD and Pharma wanted me muzzled.
Everyone who heard my Tetragrammaton podcast with Rick and Huberman now should fully see how this system was engineered from an 80,000 kilometer view to keep mammals well.
SUNLIGHT LOWERS BRAIN INFLAMMATION BY CREATING HIGHER LEVELS OF MELATONIN AND KYNURENINE ACID AT THE SAME TIME. THIS PROTECTS MELANIN SHEETS.
IT IS ALL ABOUT LIGHT WE LIVE UNDER. SPECIFICALLY, UV AND IR LIGHT FROM THE SUN VERSUS ARTIFICAL LIGHT MADE BY MAN.
It is no longer a mystery……….it is a choice. Sound familiar? See the bottom right of the picture from 2011.
THE FINAL REVIEW
Why does the metabolism of serotonin matter so much in the melanin story? Because this reflects where the sun is in relation to the Earth in a seasonal year. For instance, the serotonin “branch” in the water fall flows at a less efficient rate in the serotonin/melatonin pathway compared to the kynurenine “branch” (~98% vs ~2%).
It also points out why exogenous supplementation of melatonin upsets the flow in this pathway because it changes the charge density of tissues like the skin and retina where melanin is located in the RPE.
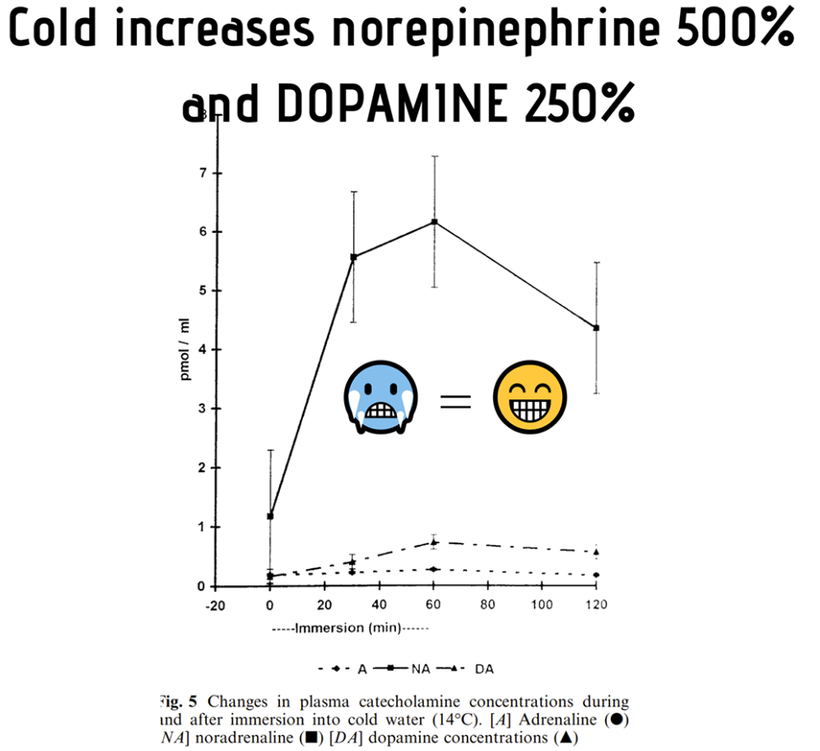
Here is the key point. A lack of sunlight or melanin degradation by any cause leads to a change in how the pathway operates in neuroectoderm in humans. Chronic inflammation results from a lack of sun for any reason. It can also happen via hypoxia, as the slides below shows because hypoxia DEGRADES melanin to adrenochrome and DOPA, noradrenaline etc………
This degradation causes a myriad of things to develop in modern humans living under artifical light such as during an infection or an autoimmune disease. Light fundamentally changes the manner in which the kynurenine pathway operates. This is why the Cold Thermogenesis series told you biochemistry was thermoplastic with respect to light frequencies. This highlights why the SCN responds to light and temperature only. This is how the brain tells seasons.
The part of the pathway that normally synthesizes beneficial molecules slows to a trickle while the floodgates open for the harmful part of the pathway.
Why does this happen?
Well, inflammation is the answer:
For the big time details I did a whole webinar on this called Kruse for Dummies. Review that there. This blog is to synthesize all the lessons I have given you.


SUNLIGHT IS THE REASON
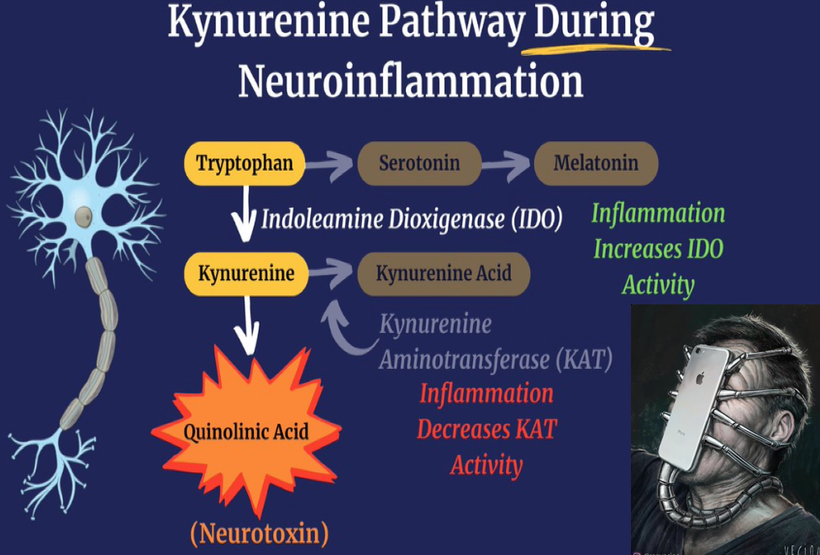
^^^ Sunlight increases the catalytic activity of enzyme IDO
Making more kynurenine and less serotonin and melatonin leads to ALL CHRONIC DISEASES and INCREASES ALL CAUSE MORTALITY
WHILE simulataneously SUNLIGHT decreases the catalytic activity of KAT
Making less kynurenine acid (neuro protective for melanin) and more quinolinic acid (harmful for melanin) from melanin degradation.
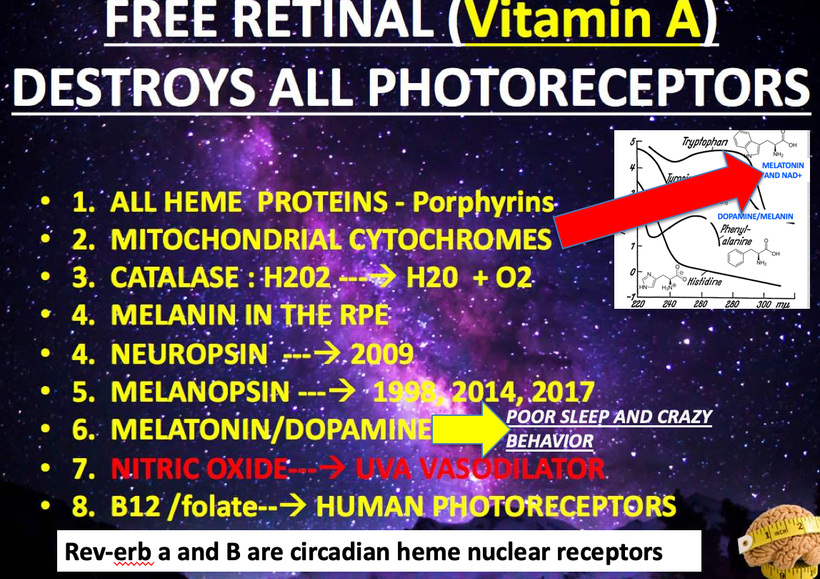
A lack of sun causes melanin degradation via hypoxia. Non native EMF cause liberation of Vitamin A from the loose covalent bond of opsins in the non visual photoreceptor system due to alien light and that Vitamin A cause hypoxia by lowering NAD+ in a cell. This is today’s major cause of disruption in this pathway.
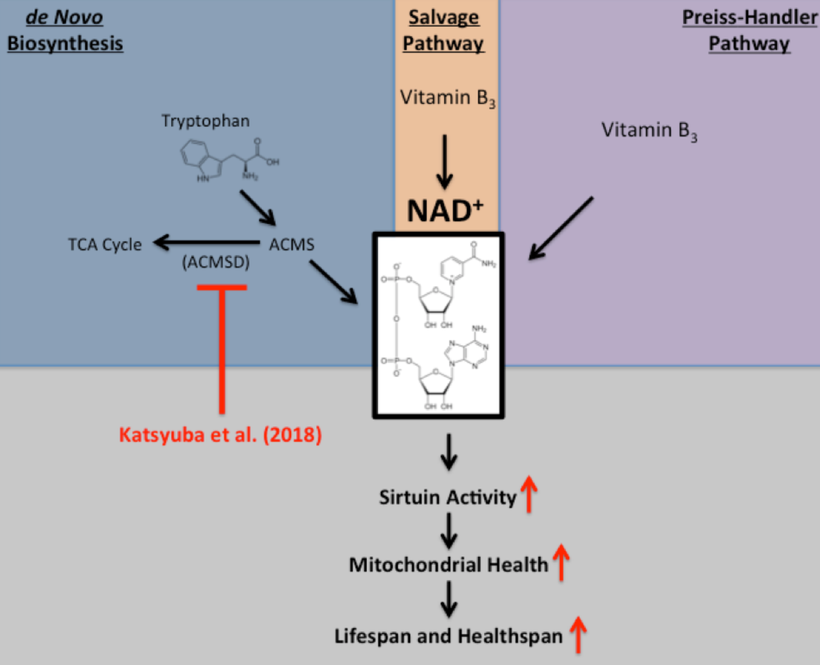
How does this happen? A lack of sun changes the catalytic efficiency of an enzyme called IDO in the pathway. This changes cytokine signaling which in turn changes the biochemistry of the pathway. Note a lack of sun or excess nnEMF is the key stimuli today. In the Neandetthal’s day fire and cold cave life was enough to shrink 125 grams of brain tissue from them.
A lack of sun increases these cytokines in neuroectodermal derivative to increase IDO activity:
 IL-1b
IL-1b
 INFg
INFg
 IFNa
IFNa
 TNFa
TNFa
 IL-6
IL-6
 IL-12
IL-12
Remember TNF alpha from the original leptin blogs of 2009? Note the date. You do not need PEER centralized science to prove nature’s recipes, when Nature’s laws are defined by E=mc^2.

While these cytokines decrease KAT activity:
 IL-1b
IL-1b
 INF-g
INF-g
 TNFa
TNFa
SUNLIGHT reduces all inflammatory markers and this highlights the strategy of Big Pharma and Bill Gates clearly now.

This is how light changes disease phenotype irrespective of genes or biochemistry. Anyone who tells you otherwise is a centralized scammer or ignorant.
Hence, persistent chronic inflammation from a lack of sun or too much nnEMF slows the production of essential neurotransmitters, neurohormones, and neuroprotective substances, steering it instead toward self-destructive processes in neuroectodermal derivatives in mammals.
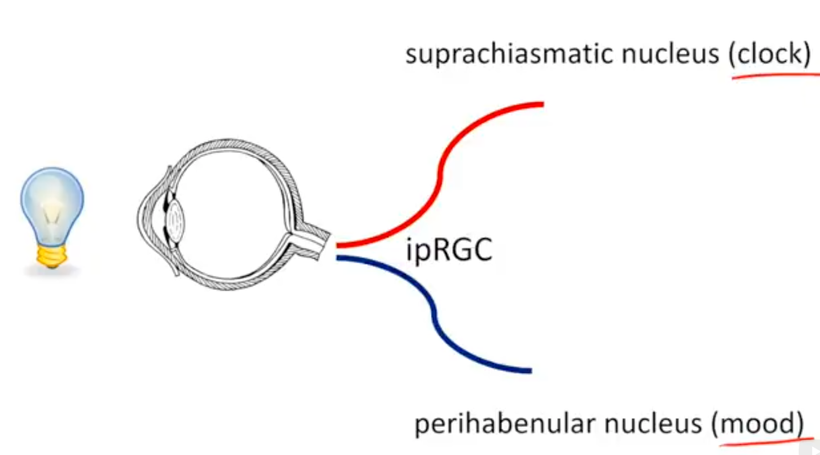
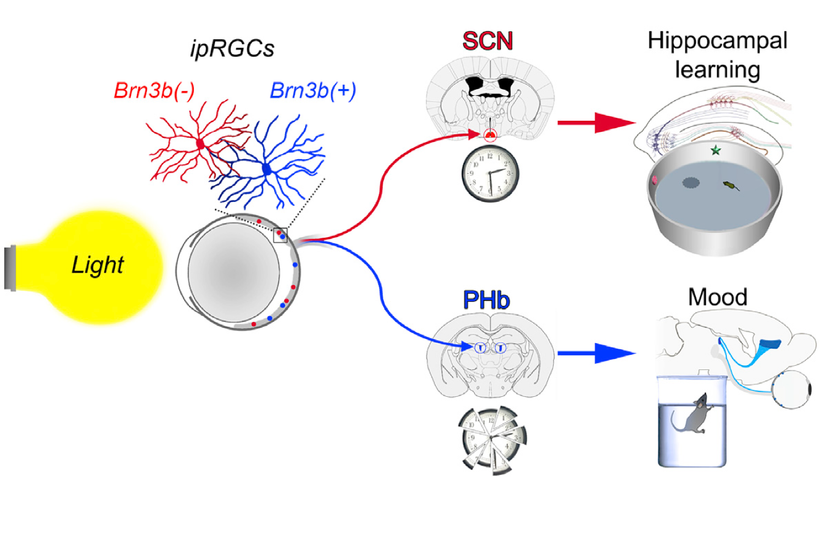
In humans we have extra neuroectoderm to protect in our frontal lobes. That photonic switch is in the habenular nucleus pictured above. When melanin is degraded in this pathway all he’ll breaks loose in executive function. These alterations eventually lead to the disruption of limbic and paralimbic brain circuits, compromising emotional functioning. This explains how light plays the leading role in the development of psychiatric symptoms associated with altered solar redox and many mitochondrial illnesses. It’s no longer a mystery. You just need to read the literature and connect the dots to POMC biology and melanin production and degradation.
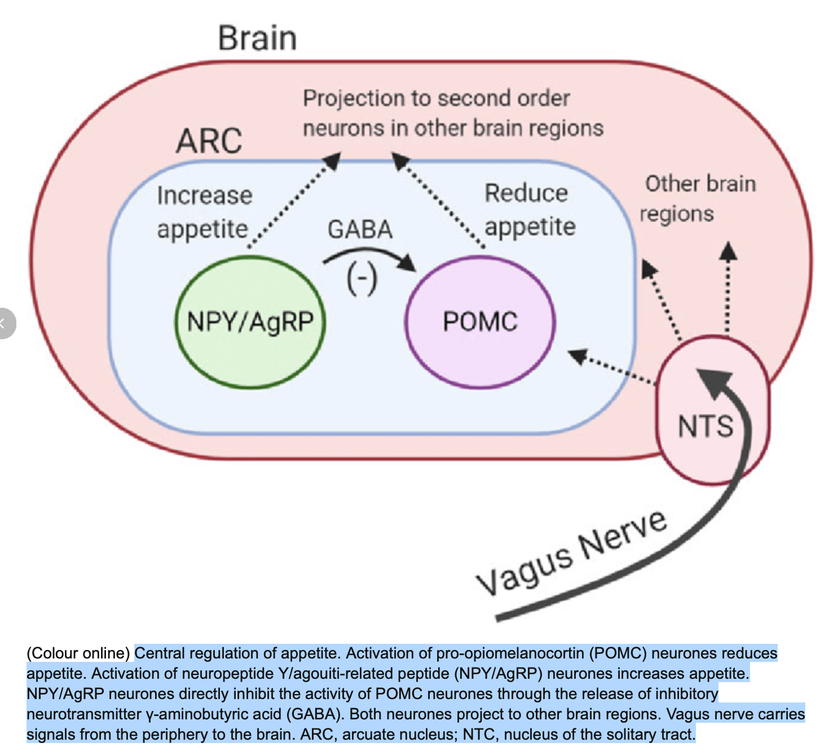
^^^^Vagal nerve stimulation increases melanin renovation. Anything that increases vagal nerve stimulation is beneficial. No one in neurosurgery realizes why when we put in a vagal nerve stimulator patients often lose weight. I learned it over 20 years ago.
MELANIN RENOVATION Rx
1. Did you know that mitochondrial melatonin protects melanin from destruction by way of the NRF2 pathway? This is a critical link in the melanin renovation Rx. When you believe in yourself, in your innate abilities to do and to think, you know implicitly that anything is possible. The impossible even becomes easy for you while everyone else seems to struggle. Time relativity changes and your life and recovery go better. AM Sunlight helps this by boosting your melatonin levels to improve your colony of mitochondria and its ability to deal with Nature’s diurnal variations in light.
Melatonin/melanin protects the skin from the deleterious effects of UV radiation as well as a plethora of other cells against oxidative stress. Melatonin made endogenously has hundreds of metabolites that protect us by acting on the immune system and NRF2 inflammatory pathways to keep us well.
In this way melatonin and Vitamin D3 are similar. Their ENDOGENOUS metabolites are as important as the parent hormone. The use of exogenous supplements either lowers the metabolites and parent hormones in our body. Remember this effect. Supplements ruin fidelity.
Exposure to Ultraviolet Radiation in the A band promotes the expression of melatonin receptors in the skin via alpha MSH made from POMC. This occurs via melatonin receptors called MT1 and MT2.
In the lab, pretreatment with melatonin reduced cyclobutane pyrimidine dimers (DNA strand breaks) levels by approximately 40%. Remember life is not lived or built in a LAB……….Nature is your lab. AM sunlight increases melatonin and melanin. https://www.nature.com/articles/s41598-017-01305-2

2. There are many paths to melanin renovation but all of them have to support tyrosine pathways over phenylalanine pathways, the use of sunlight, and augmentation with pulsed IR-A light at a specific frequency.
The key to melanin renovation is solar light exposure that has IR-A full spectrum exposure and UV-A exposure in combination.
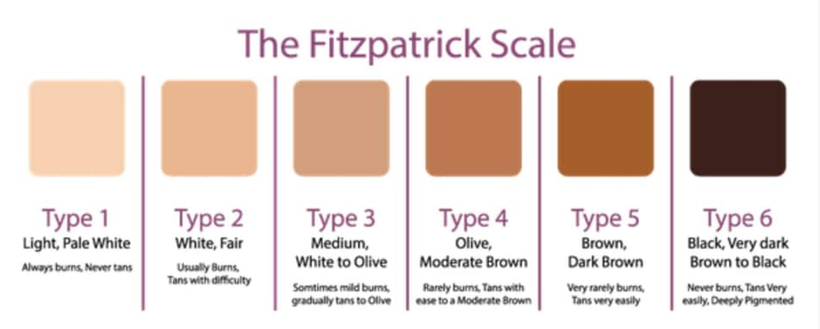
Type 1 skin One hour of IR-A and UV-A exposure over a day
Type 2 skin two hours ” ”
Type 3 skin three hours ” ”
Type 4 skin four hours ” ”
Type 5 skin five hours ” ”
Type 6 skin requires 6-8 hours of exposure
FOR PULSED LED IR-A light exposure on atrophic skin in my clinic I use a pulsed red frequency at 4 different frequencies in my clinic based on the patient’s pupillary exam and retinal exam. If I do not examine you I cannot tell you your answer. This treatment is done for my personal clients based on my own neurological exam. This allows me to add more light via their optic nerve than they can get due to their skin condition. I also use this for people who have previous eye surgeries that hinder their melanin renovation.
There are 3 dominant phases of synchronized metabolic and transcriptional reprogramming in pigmenting the skin. The melanogenic trigger is associated with high MITF levels along with rapid uptake of glucose by the cell. Blue light exposure stimulates massive glucose mobilization from ACTH from POMC. The transition to pigmented state is accompanied by increased glucose channelization to anabolic pathways in biochemistry that support melanosome biogenesis. The H+/D optical switch you heard about in the Kruse for Dummies talk is critical in optimizing this step.

This expands the cell pool that can pigment with melanin. As a result, SREBF1-mediated up-regulation of fatty acid synthesis results in a transient accumulation of lipid droplets with an enhancement of fatty acid oxidation through mitochondrial respiration. I believe this is how we create VUV endogenous in our mitochondria in 2024. While this heightened bioenergetic activity is important to sustain melanogenesis, it impairs mitochondrial efficiency as time elapses. This means as we age or heteroplasmy rises we need more sunlight not less.
Timing is critical to melanogenesis. The SCN timing mechanism is the key clock manager in this process. It controls the biophysics of the skin and the lipid rafts controlled by cholesterol fractionations in cells. This is how mammals control their optical windows to light. HDL cholesterol upregulates melanization and LDL cholesterol puts a limit on it. This implies lipid chemistry is also a seasonal part of biochemistry that the centralized paradigm whiffs on. High LDL cholesterol is the signiture of light stress and it leads to atrophic skin! Blue light exposure causes this light stress response most often. UV light lowers LDL and APO B. In fact, every lipid the centralized lipidologist link to cardiac disease and PAD are lowered by sunlight.

Pater Attia and Rhonda Patrick are not experts on lipids when you understand what I said above. In fact, I will tell you their advice is going to harm people in the modern world. The picture above shows why. This twitter thread is a dagger to their food guru and biochemistry centralized hearts. Reject that centralized thinking in 2024. This thread has been pinned to my Twitter profile page. READ IT, because Attia, Patrick, Wolf, Sirtoli, Goodrich, Huberman, and anyother critic of my work are not. And if they said they did then you really know they are just not smart enough to hire.
https://twitter.com/DrJackKruse/status/1659534650899853314
What else does this story imply?
This implies that if you cannot use the TCA cycle well, you will not tan well either. This is why cancer patients often have atrophic skin that is pale even when they are placed in the sun. They have redox shifted their colony of mitochondria out of their ability to tan. The redox shifting the metabolism moves human biochemistry toward glycolysis and away from lipid synthesis. The driver of the shift is the change in the binary code of H+ and D in the matrix I spoke about at length in the Kruse for Dummies lecture. This carburetor action changes biochemical efficiency via the kinetic isotope effect (KIE). The recovery phase or photo repair part of the melanin cycle is accomplished by surface 380 nm light exposure (and via the eye) and is accompanied by activation of the NRF2 detoxication pathway. This step requires a properly functioning matrix moving H+ to NAD+ away from NADH and NADPH. Melanin detoxs everything in your body. If you are being sold detox ideas fire your clinician and go south with the money.
NRF2 detox pathway only is tapped by redox power.
If your cells lack the net negative charge your cells never experience this pathway.
SCIENCE GEEKS: Nrf2 regulates important genes such as heme-creating heme-oxygenase 1(HO-1), glutathione S-transferase (GST), glutathione reductase (GR), glutamate-cysteine ligase subunits (GCLc and GCLm), NAD(P)H quinone oxidoreductase-1 (NQO1), and thioredoxins (TrxR1), among others. Nrf2 stays sequestered in the cytoplasm by its interaction with the Keap1 protein complex (Keap1-Kelch-like ECH-associated protein 1). When an oxidative stressor or electrophile interacts with the Keap1-Nrf2 complex, it allows for the release and subsequent nuclear accumulation of Nrf2 through a complex process of ubiquitination-inhibition, thus triggering an anti-stress response by the cell. Now you know why you had a huge ubiquitination series years ago. Ubiquitination turns on and off protein synthesis which makes up half of the semiconductive frame in mammals. Water is the other half.
Inhibitors of lipid metabolism can resolve hyperpigmented conditions. The mitochondrial matrix is critical in creating fatty acids that do this!! This is why cholesterol biology is a gatekeeper of melanogenesis and saying statins are part of a longevity practice is PURE INSANITY and shows a lack of fundamental understanding. Abnormal cholesterol pathways in the skin and blood are always found in people who are solar deficient and who live indoors. These are the people who often develop metabolic diseases. Diabetes is the major worldwide disease that characterizes this idea. Patchy cutaneous hyperpigmentation is associated with comorbidity in more than 30% of patients with diabetes and obesity. (acanthosis nigricans)
Fatty acids are essential for cell survival as they serve as key structural components of cell membranes and important signaling molecules. Fatty acids are also the most calorically dense form of energy storage with the conversion of excess glucose into fatty acids protecting against glucotoxicity and providing a much larger energy reserve than glycogen for times of nutrient scarcity. Given the vital roles of fatty acids, cells have evolved mechanisms to maintain them at adequate levels using solar light frequencies as the gatekeeper. This includes mechanisms to take up exogenous fatty acids but also to generate fatty acids from alternative carbon sources through a series of enzymatic reactions, a process highly conserved across all phyla known as de novo lipogenesis (DNL).
There is a reason Vitamin C does what it does in the brain where melanin is located. Do your experts understand it? When melanin is degraded glutamate is released and this changes the biochemistry in the region to preserve endogenous melanin sheets. Vitamin C is a cofactor in all the metabolites made from melanin like dopamine, noradrenaline, DOPA, and epinephrine. No seems to see how or why it happens. Now you do.

Ascorbic acid (vitamin C), an antioxidant vitamin, plays an important role in protecting free radical-induced damage. A previous study has shown a decrease in basal vitamin C levels in type 2 DM patients. Vitamin C actually inhibits melanogenesis in humans with no defects in circadian signaling or who have altered cholesterol biophysics at the skin level. Vitamin C is structurally similar to glucose and can replace it in many chemical reactions and thus is effective for the prevention of nonenzymatic glycosylation of protein. The supplementation of vitamin C usually will improve blood glucose level management and lower glycosylated hemoglobin (HbA1c). If it is used too long by a human, it will inhibit melanogenesis.
This is the braking mechanism mammals have used at the end of the summer to change from their summer coats to their winter coats. Today, humans have ruined the fidelity of this system with modern lighting and nnEMF use for communication.
Vitamin C donates electrons to semiconductive proteins like collagen and cholesterol. This changes their charge. The change in charge is how the animal knows the seasons are changing. Most mammals that come to see me are in a constant light-stressed environment with high LDL cholesterol and atrophic skin. Their skin needs to be moved to the proper seasonal coat when they come to see me. Vitamin C lowers blood glucose. Vitamin C is a tool we can use to facilitate the changing of the guard in their skin. Vitamin C is easily oxidized, participating in the redox systems of the body. It is essential for the synthesis of collagen, elastin, and cellular substance in the epithelium and also prevents the formation of excess free radicals. The impairment of collagen synthesis causes loss of skin firmness, the appearance of wrinkles, and capillary brittleness. Vitamin C determines the healing of traumatic lesions and burns.
It also participates in the synthesis of tyrosine and phenylalanine and affects pigment changes. In people with skin or ocular albinism, I have used Vitamin C and IR-A light to pretreat the skin for melanin renovation.
Other key things to be mindful of during a melanin renovation:
AVOID USE OF THESE THINGS
1. MAO inhibitors
2. Chocolate
3. Red wine
4. Ginger
5. Tattoos
TATTOOS
The skin is your largest organ and it is a massive solar battery for your brain. When you think about tattoos think about burns. How they calculate burn areas is how I look at tattoo coverage in terms of medical risk.
The scientific consensus is that the entire, three-layer organ makes up about 16% of a human’s weight — equal to about 20 pounds for a 125-pound person.
If your mom warned you that your ink is forever when you announced your intention to get a tattoo, she was right. But not for the reason you might think. While common wisdom has it that tattoo ink bypasses the permeable top layers of the skin and remains embedded in the dermis, the second skin layer, the real truth is a bit more complicated — and more interesting.
Melanin normally clears the skin of heavy metals via chelation in ink so this degrades the melanin in your skin but the latest research is a bit more concerning. Recent research has revealed that macrophages, a type of white blood cell that specializes in gobbling up invasive pathogens, mistake tattoo ink for an infectious cell and flock to the scene to protect the body from the foreign substance. They show up, encircle the ink, and process it. These cells are critical in cancer and autoimmunity development.
Dendritic cells (DC) are a class of bone‐marrow‐derived cells arising from lympho‐myeloid hematopoiesis that form an essential interface between the innate sensing of pathogens and the activation of adaptive immunity in our skin. These cells differentiate into cCDs. These cells are chronically replaced from bone marrow in tattooed patients. This chronic stimulus is a stem cell stressor in the skin and marrow. This increases aging in those two organs and makes them less efficient in their functions. Tattoos do not help a brain think well.
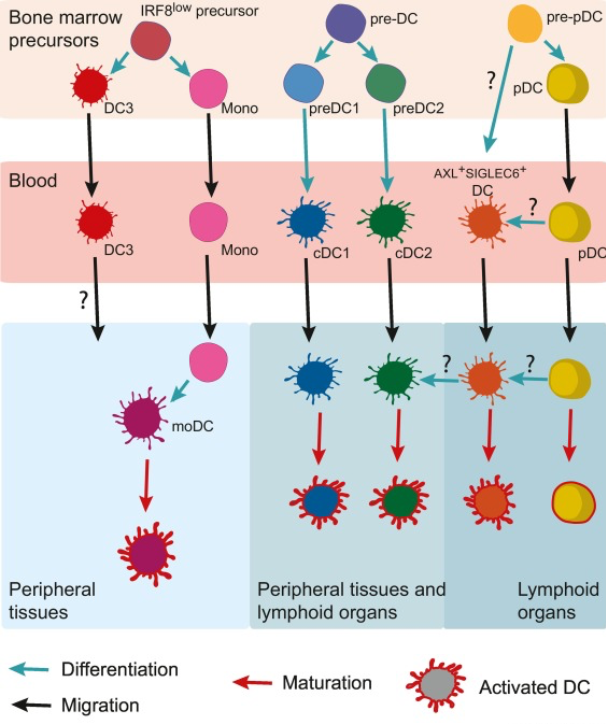
Dendritic cells (DCs) are key orchestrators of immune responses in humans. A specific DC subset, conventional type 1 DCs (cDC1s), has been recently associated with human cancer patient survival and, in preclinical models, is critical for the spontaneous rejection of immunogenic cancers and for the success of T cell–based immunotherapies. The unique role of cDC1 reflects the ability to initiate de novo T cell responses after migrating to tumor-draining lymph nodes, as well as to attract T cells, secrete cytokines, and present tumor antigens within the tumor microenvironment, enhancing local cytotoxic T cell function. Strategies aimed at increasing cDC1 abundance in tumors and enhancing their functionality provide attractive new avenues to boost anti-tumor immunity and overcome resistance to cancer immunotherapies.
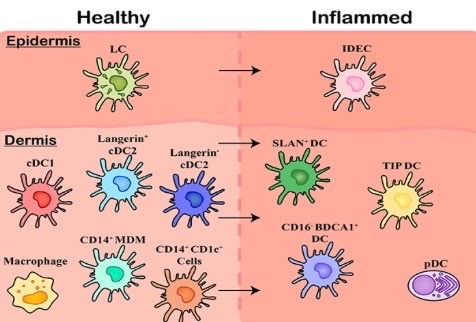
Within healthy skin, it is believed Langerhans Cells (LCs) are the solitary population, however, the underlying connective tissue of the dermis contains a range of subsets including cDC1, cDC2 (langerin expressing and langerin negative populations), and CD14 expressing cells including tissue resident Macrophages, Monocyte-Derived Macrophages (MDMs), and an uncharacterized CD1c+population. Upon inflammation, these cells are also present however a number of other subsets of MNPs migrate in, including Inflammatory Dendritic Epidermal Cells (IDECs) in the epidermis. This is why I knew to look for a T-Cell lymphoma in this tattoo. Note how atrophic the skin was from a lack of melanin. I mentioned this in the Tetragrammaton podcast but it hit the floor in edit room.
If macrophages clean up the ink, and the immune cells aren’t immortal, then why do tattoo markings last so long? Scientists asked the same question and mouse studies led to an eerie answer: Once the macrophages die off, even more macrophages have to be recruited from the bone marrow to swoop in, eating the ink their dead brethren once contained. This generational turnover means tattoo ink can last for years with minimal fading — and keep Mom’s name intact for a lifetime.
Your immune cells, bone marrow, and melanin in the skin may never recover. This means the circadian mismatch from the skin issue has had a massive effect on the heteroplasmy rates of the gut and brain for years. How much of the body covered is huge and what parts of the body are also germane to this because not all skin patches on humans are equal because POMC translation varies in the skin of humans.

WHAT TO DO IF YOU ARE IF YOU ARE Tatted up you need to consider a couple of hacks to try to offset the risk:
1. DHA upgrades to your diet to improve macrophage function helps offset some of the problems tattoos bring to our skin because DHA is metabolized to maresins, protectins, docosanoids, and elovanoids to keep the immune functioning upregulated in the skin. This lowers the inflammation to improve the optical density to allow light to get to RBCs and the blood. Once melanin is present it will chelate and remove any mobile heavy metals from tattoo ink.
2. I like the use of myrrh for those with tatoos directly on the tatted region.
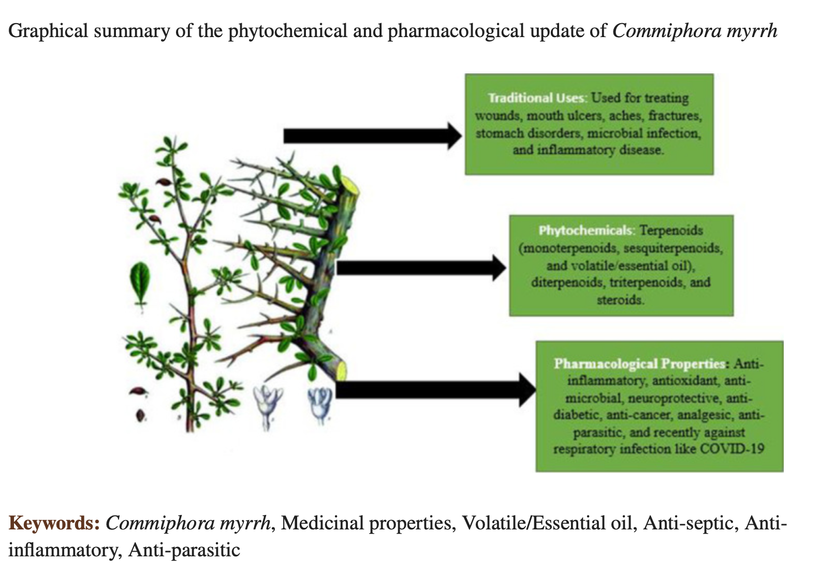
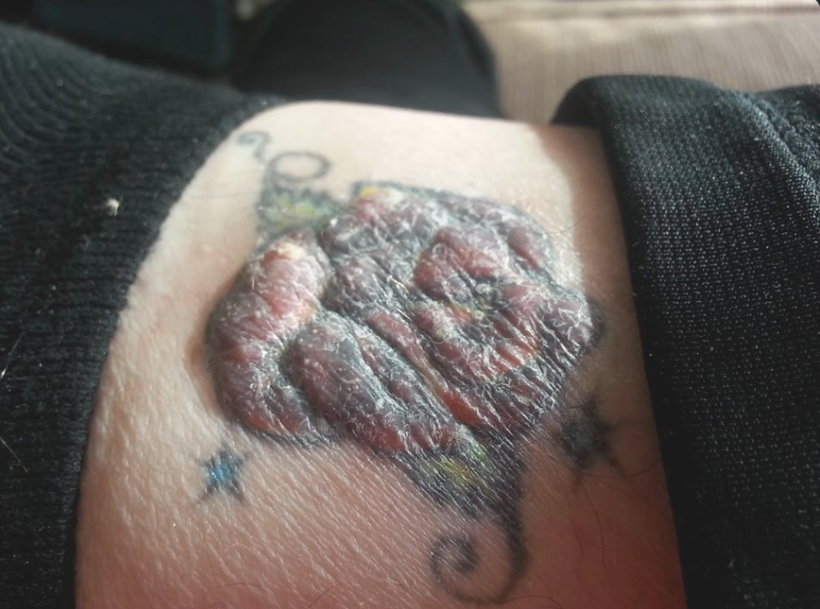
The prized resin of myrrh is stuffed full of substances that have anti-inflammatory and antimicrobial properties, which may also have improved the look of the skin. Myrrh also can increase red light exposure to tattoo skin because of the effect of terpenes. Myrrh is something I have my professional athletes use to offset the Tattoo risk for CTE, neurodegeneration, and concussions. This by no means lowers the chronic risk of the tattoo but it can help in the short term when you are suffering from a health deficit related to the skin (below as an example)

3. Consider laser removal only if certain areas that have ink become affected by disease. If you develop aggressive PAD, CAD, or neurodegeneration then I offer my patients removal options.
ARTHRITIS AND MELANIN
There are cases in the literature where a high dose of Vitamin C can be used in inborn errors of metabolism of the two amino acids that help create melanin from alpha MSH. This is where I got the idea to use this treatment in albinos or in people with atrophic skin. For example, alkaptonuria is a rare inborn error of tyrosine metabolism in which deficient activity of homogentisic acid oxidase produces an accumulation of homogentisic acid and leads to debilitating ochronotic arthritis in adulthood.
The administration of relatively large amounts of ascorbic acid to the adults has been done and published and the results showed a disappearance of benzoquinone acetic acid from the urine of these patients. Interestingly the level of excretion of homogentisic acid did not change.
These papers were highly influential for me in pushing melanin renovation in people who get rapid onset osteoarthritic symptoms and live in a high-density 3-5G environment. Right now this is the biggest problem I see in my own private clients since 5G has been turned on in the USA.
A lot of unnecessary orthopedic and neurosurgical procedures are being done on people because this information is not well-known to centralized providers.

What is ochronotic arthritis? Due to the homogentisic acid oxidase deficiency, homogentisic acid accumulates in cartilage/tendons and connective tissues which leads to ochronotic arthritis. If you do not know about it you’ll never test the urine for homogentisic acid. This is a sign the NRF2 pathways are turned off due to a lack of redox power.
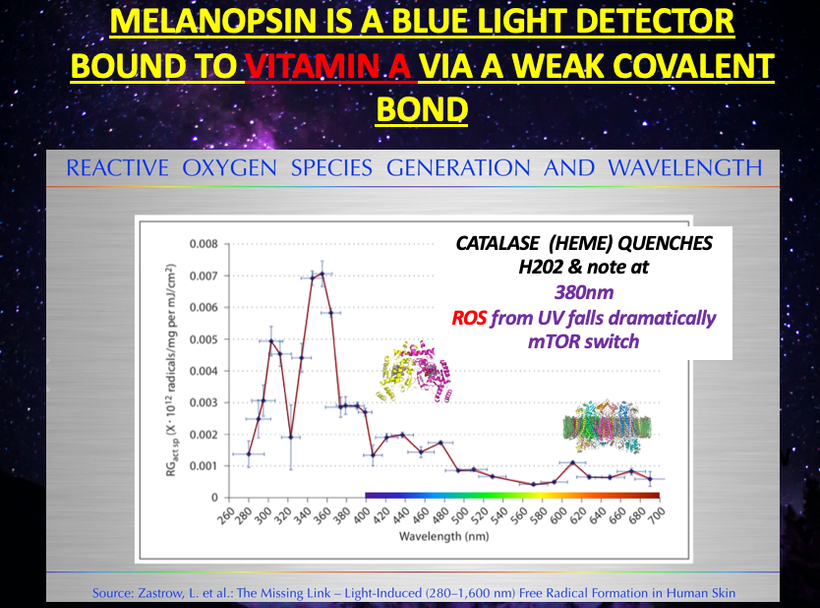
The slide below shows you we need a ROS light stimulus from the sun’s UV rays, to stimulate melanin production. Thirs is why 380nm is critical. It is also why dermatologist do more to harm human longevity with their centralized advice.
This implies to you that not all radiation is problematic humans. I believe melanin renovation is one of the better nnEMF strategies one can employ. Most people with EHS are quite pale.

The key to the diagnosis is the rapid onset of the symptoms and the zip code of where the patient lives. Centralized orthopods will recommend physical and occupational therapy initially. They do this to help maintain muscle strength and flexibility. Analgesic treatment (NSAIDs) is believed to be essential for joint pain at an early stage of ochronotic arthritis, but in my experience, orthopods will push rapid joint arthroplasty as their go-to treatment for significant degenerative arthritis when needed. Decentralized MDs need to know that a lack of tyrosine from nnEMF exposure can cause rapid-onset arthritic changes. The best plan of action is high-dose Vitamin D, DHA, and relocation to a strong UV environment for a period of time to renovate melanin. Getting out and staying out of the 3G-5G environment is mandatory for success. Why? See the slide above. nnEMF turns off melanogenesis. It also ruins the fidelity of the clock genes that control this very sensitive dance in the lipid rafts of your membranes. People who get white spots all over their skin when they use the sun are getting a signal from within that their cholesterol rafts are not optimized by light in proper topological fashion.

KEY BLOG MOMENT: nnEMF causes more destruction of melanin than anything on Earth today in my opinion. all nnEMF cause hypoxia and hypoxia is the fastest way to destroy melanin production. This is why cities with electromagnetic pollution often are filled with pale people with low Vitamin D states. These people will also be afflicted by EHS, mold, Lyme disease because of a chronic lack of melanogenesis mimicks pathology of these diseases. I do not believe you can get well until you reduce the dose of radiation and add back in the right radiation that stimulates melanin production. That is UV-A light around 380nm. The blue light hazard really destroys melanin between 400-550 nm light. It does the same thing to melatonin.
You do not need to have an inborn error of metabolism to get this problem. Normal people can develop this tyrosine problem post wireless/blue injury in high-density EMF areas. Many cases of electrohypersensitivity are actually this disease and they go undiagnosed or misdiagnosed for decades.

NOT ALL RADIATION IS EQUIVALENT IN THE ELECTROMAGNETIC SPECTRUM
This is critical for the skin and brain which are neuroectodermal derivatives.
All UV light stimulates POMC to make alpha MSH. Do not forget it. Some parts of the UV spectrum, however, are better than others at creating melanin. UV-A light is the sweet spot for mammals. This is the radiation we crave and need to get to optimal. Hence this is why Nature built cells to be addicted to sunlight via beta-endorphin in POMC cleavage. It turns out UV-A light is best at creating a tan. Guess why?
Radiation along the electromagnetic spectrum is not always a death sentence. It can be hormetic when it falls within the visible spectrum of light. Radiation from this spectrum has a hormetic effect on DNA repair in sublethal doses that we normally get from Nature. The key is getting the dose right and the exposure time right for a cell. Low doses of radiation can stimulate DNA repair through the activation of four transcription factors, PARP-1, PARP2, ATM, and Ku70. It may seem counterintuitive and go against the conventional wisdom you have heard, but this is how nature acts in reality. With low-dose radiation, Ku70 activates a DNA pathway called non-homologous end-joining repair. This is how DNA repairs single strands and double-strand breaks it normally faces in life. The sun’s EMF is hormetic for life’s DNA. Nothing else in the electromagnetic spectrum has this ability, and that is a big modern problem.
Many do not know ALL mammals are capable of direct photo repair. I call this process melanin regeneration. You can see a picture of this above.
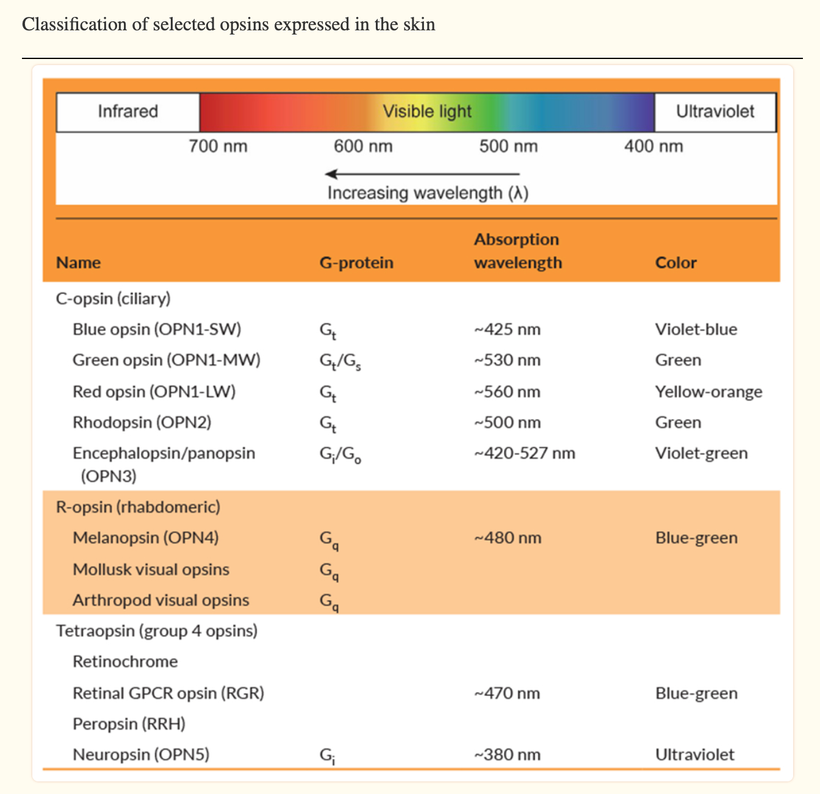
Russell van Gelder, M.D., Ph.D., a professor of ophthalmology at the University of Washington, studied the circadian rhythms of genetically tweaked mice that were missing rod and cone cells and melanopsin. As expected, the circadian rhythms of these mice continued cycling but could no longer adapt to changes in light exposure in the visible spectrum.
Surprisingly, though, the activity patterns of their retinas were still responsive to light changes, suggesting that there was another light photoreceptor in play in the eye and skin. That pigment was neuropsin.
Neuropsin is a UVA light detector protein in our skin and cornea that is optimized for 380nm light.
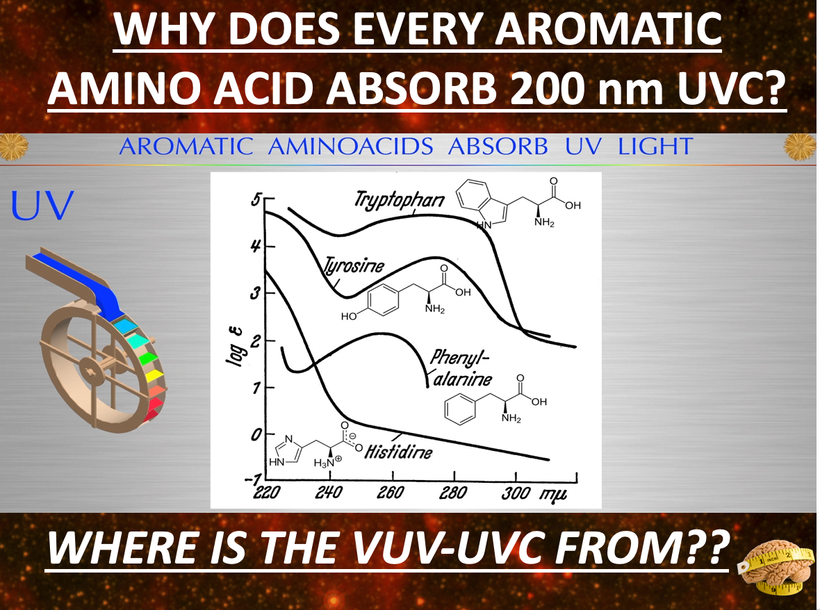
Did you know leptin absorbs best in the UV spectra at 220 nm? Do you think these facts aren’t connected?
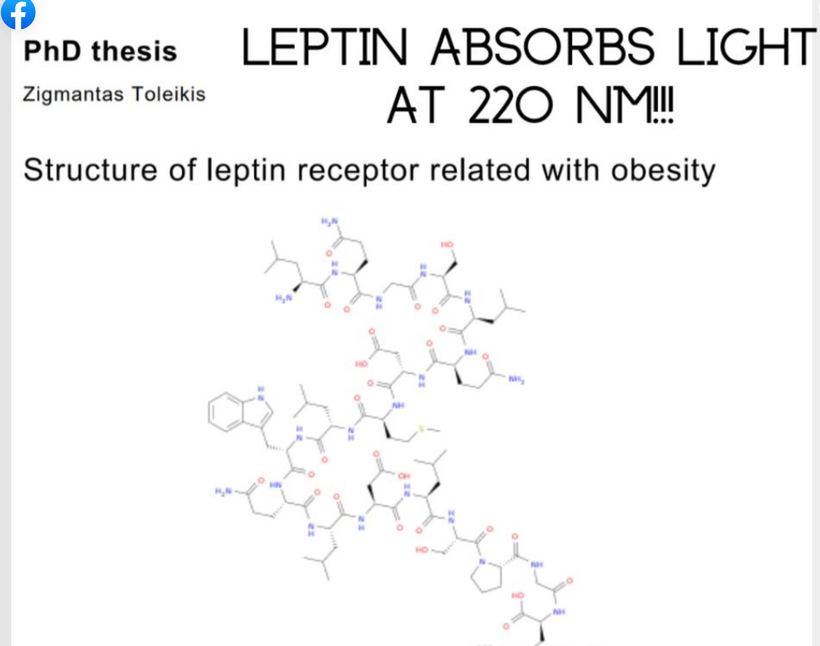
Neuropsin is the afferent loop of this light reflex and works with sunlight at 380nm and leptin is the efferent loop of the photo repair mechanism that responds to UV-C light made by our endogenous wide band gapped semiconductors inside of cells.
This opsin, like all opsins, works with the Vitamin A cycle in the body. Most people struggle to convert dietary carotenoids into the active form of Vitamin A needed for visual photo-reception if neuropsin is blocked in the skin/eye. It turns out that blue light toxicity on the eyes or on the skin also ruins the conversion of dietary carotenoids to retinol and this causes circadian phase delays that underpin the mitochondrial diseases mentioned.
This will directly affect POMC creation in the eye because all of the receptor systems for POMC use Vitamin A. Neuropsin is the UV detector that is the critical signal in the eye to create POMC translation inside of our tissues where the POMC gene lays dormant. It is only transcribed by UV light release. POMC creation is known to be upregulated by UV radiation.
This opsin discovery is when I began to realize all I was taught about the sunlight in medicine might be a fallacy. The current paradigm in ophthalmology says that UV light is really bad for the eye and skin. Well, if that were true why would Mother Nature have put a UVA opsin in the cornea and in the skin?
Neuropsin has been found in the cornea of the eyes, skin, and subcutaneous fat now mimicking the exact spots where melanopsin is found. If it is blocked for any reason at all, the optical signaling for photo repair is no longer operational in cells.
I currently believe neuropsin was selected for by the KT event in eutherian mammals that made it through the last extinction event. During this time it is believed that photosynthesis was disrupted for several decades to 1000 years. This would have lowered the quantum yield of sunlight on the entire planet. A lowered quantum yield of sunlight would have sharply lowered UV light from the mammal’s surfaces and this would have driven melanin internally into their bodies looking for a new source of UV light. Here the melanocytes would have stopped when they found cells that created it. When melanin was created there it would have been used to charge separate water into H+, oxygen, and electrons to be used by mitochondria.
The return of UV-A light on the surface of Earth would have been a newsworthy event or stimulus to mammalian cells. I have a sense this is why mammals added neuropsin to their surfaces to communicate with leptin and melanin sheets now ensuring their survival today.
Mammals that survived would have needed some way to get environmental signals to their pituitary gland to drive the thyroid hormone cycle needed to drive seasonal changes and control reproduction. Without these two factors, no mammals would have survived very long, in a low-quantum yield environment. In today’s world, we now see the same similarities in our environment but for different reasons. Humans no longer live under sunlight. They live under manufactured tech light. As a result, in humans, thyroid diseases and fertility changes are now at pandemic levels because of a lack of photo repair and increasing hypoxia at the cell level. (380nm)
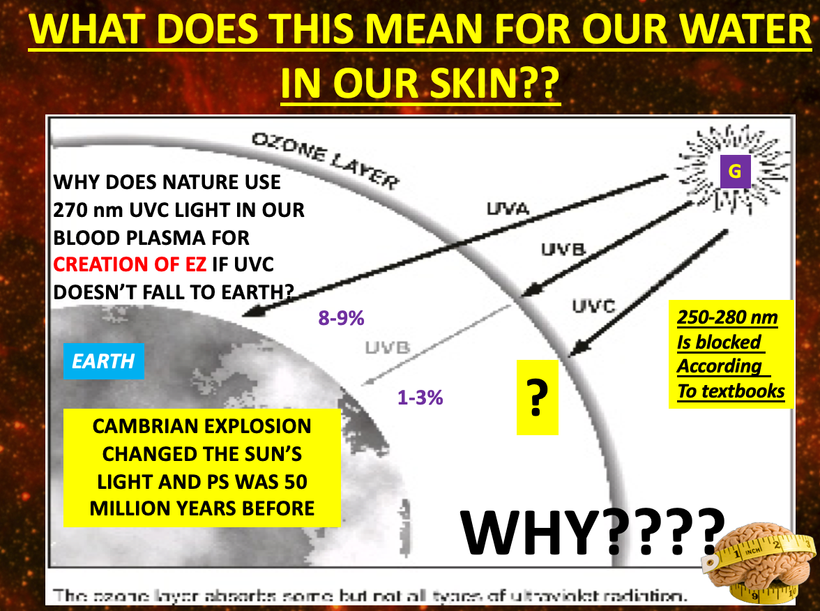
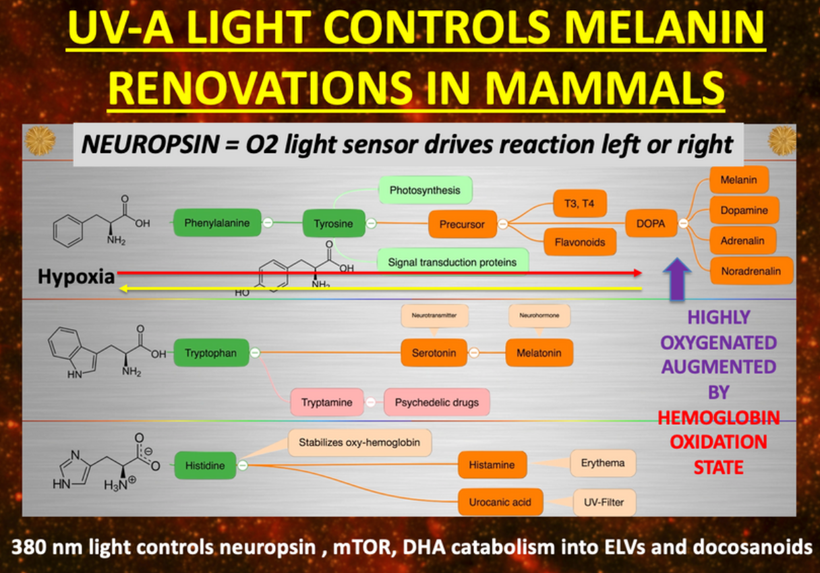
I believe that UV-A light is critical in driving thyroid cycling in all modern mammals including humans. It also is linked to POMC creation and cleavage. When mammals are hypothyroid this alters POMC expression and cleavage. When we see massive spikes in hypothyroidism, it is a sign that there is a global lowering of the quantum yield of sunlight for some reason. 65 million years ago it was debris from an asteroid that cause a blockade of specific frequencies of light. Today, it is non-native EMF in the ionosphere that is destructively interfering with sunlight that falls to the Earth’s surface. This explains why we have a global Vitamin D3 pandemic and why we see massive spikes in hypothyroidism and infertility. Free T3 is needed for fertility (leptin) and for peripheral nerve function. T3 is stimulated by oxygenation. Low T3 is driven by a lack of oxygen. Pain thresholds in mammals are also controlled by this reflex. (pic below)
Low free T3 levels also are associated with chronic pain and severe alterations in cardiac function. Free T3 levels have been studied in post-heart surgery patients and have been found to be a very good reliable outcome measure. Free T3 tends to trend with POMC, melatonin, and dopamine levels because both need UV light exposure to recycle themselves in mammals. They are all excellent oxygen sensors too as is hemoglobin. Low free T3 levels are a sign of low UV light exposure and low tissue oxygenation. This affects melanin semiconductive sheets inside our skulls and on our skins. Note the slide carefully again. A lack of UV light decreases the electronic storage of energy in our cells.
Thyroid hormones are made in the anterior pituitary gland in response to light frequencies from the sun. But as the slide shows above they can be made from melanin sheets when they degrade too.
Also, both the thyroid hormones triiodothyronine (T3) and thyroxine (T4), which have long been appreciated to alter skin physiology, including human hair growth and pigmentation, and thyrotropin stimulating hormone (TSH), for which human hair follicles have recently been identified as a direct target, often act synergistically with leptin in enhancing mitochondrial energy metabolism similar to the thyrotropin-releasing hormone, the factor that triggers TSH secretion in the hypothalamus. This is why sweating and hair growth return were listed in the original Leptin Rx blogs. Furthermore, human hair follicles express prolactin, which regulates hair follicle cycling, while systemic prolactin levels are recognized to render target tissues refractory and resistant to leptin-mediated effects
Leptin and its receptor are also expressed by human hair follicles. POMC is present in all human hair cells. When hair is lost by mammals you know this pathway is defective at some level.
The human skin expresses the genes for corticotrophin-releasing hormone (CRH) and pro-opiomelanocortin (POMC). The cutaneous CRH/POMC expression is highly reactive to common stressors (light) and to the nutritional status that are key modulators of mitochondrial energy metabolism. Therefore, studies on the interaction of leptin and CRH/POMC signaling in their regulation of skin and hair physiology and pathophysiology should have a high priority for clinicians. Today they are ignored by the centralized paradigm. They aren’t by Uncle Jack’s army of doctors.
It is believed in 2024 that leptin is predominantly synthesized in adipocytes, including subcutaneous adipocytes. However, the synthesis of leptin and its receptors has also been detected in human and mouse fibroblasts and keratinocytes. In fact, leptin and its full-length receptor are present in the human epidermis at the gene and protein levels. This makes a ton of sense when you think about what neuropsin is doing with leptin. It is the solar light reflex that drives ALL regeneration programs in mammals. I wonder when Micheal Levin will wake up to this?
Leptin and leptin receptor expression in the human skin was validated and confirmed by real-time quantitative PCR. It is no longer a good guess by Uncle Jack, it is now a known fact.
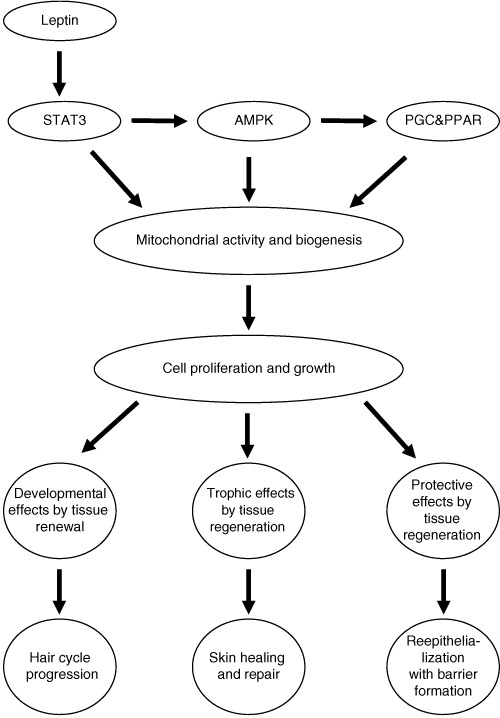
THE BIRTH OF THE LEPTIN Rx 20 YEARS AGO
This is the sciency part for my skeptical profession who mocked me 20 years ago as a quack: Numerous studies have now unequivocally confirmed that leptin and STAT3 are linked to cell differentiation, proliferation, migration, and survival in the skin with pronounced effects on angiogenesis, blood flow, and tissue perfusion. Leptin is a potent modulator of innate and adaptive immunity and upregulates POMC in the skin and antimicrobial defenses in human skin, e.g. by stimulating the expression of human β-defensin-2 expression.
Leptin has also been shown to induce the expression of interleukins in the skin, particularly that of interleukin-8 (IL-8). The diversity of leptin-dependent signaling in the skin is illustrated by the fact that the hypoxia-inducible factor-1α, which controls the expression of multiple different genes, including that of key regulators of angiogenesis and wound healing, is also upregulated by leptin signaling.
Amazing huh you all missed this party I found in a fable?

The SCN is the metronome of biology & it loses accuracy via periodicity as PER2 drops. When PER2 drops so will POMC transcription on all human surfaces. This degrades all melanin semiconductors. Melanin will need better oxygenation delivery to it to renovate itself to keep high-level optical precision. This pressures hemoglobin to function in the circulatory system. This changes our optical abilities because the dielectric constant in our tissues drops from 160 to 78. This effect is 100% tied to changes in water chemistry made by mitochondria exposed to our endogenous light. This constant changed because charge density changes in cell water. due to a change in solar power. This means we are less able to use sunlight and store it in our tissues. When this happens POMC is downregulated and melanin production stops. It stops because UV light is the ONLY stimulus to mitosis in the cells that create it. If cells cannot divide, because mitogenic radiation is absent endogenously, melanin cannot be made or renovated.
PER2 is critical in controlling the optical periodicity of the mechanism. Poor sunlight, darkness, and geoengineering with suncreams will have a major effect on cellular hypoxia via lowered sunlight. Cell hypoxia is controlled by the hypoxia-inducible factor (HIF-1alpha). Do you know the link of HIF 1 to the sun? Do you know the link to leptin?
Here is the link: HIF-1 belongs to the same protein family as the light-inducible circadian core protein Period 2 (PER2) Here is your proof sitting in your literature you never read much less connected to this story——-> (Liu et al., 2012). If I could see so should you have? We have the same training.
HIF-1-alpha is upregulated by leptin signal and solar exposure.
When you’re a mitochondriac you must begin to investigate whether hypoxia- and HIF1A-PER2-dependent pathways might regulate SIRT expression under hypoxic conditions to show why light trumps food in the controlling flux of the TCA cycle. I did look and realized something epic 20 years ago. This is how the Leptin Rx was born in my mind 20 years ago………….. But I saved the best for last.
PHOTOREPAIR
Photorepair is well known in the biophysics literature but few understand it. Not even the great physicist Fritz Popp understood it. Photorepair occurs at 380 nm in the UV-A spectrum and links to maximum oxygenation pressures in tissues
This is why UV-A light stimulates a tan in humans best. It drives the top line of the slide on the creation of melanin above toward melanin and away from tyrosine. Nature is trying to tell us that being out in the sun when UV-A light is out is a wise mitochondriac move.

What is photo repair? It is well known from biological laboratory experiments that if you blast a cell with UV light so that 99 percent of the cell, including its DNA, is destroyed, you can almost entirely repair the damage in a single day just by re-illuminating the cell with the same wavelength of UV light that destroyed it but do it at a much weaker intensity. To this day, centralized scientists don’t understand this phenomenon, called photo repair, but no one has disputed it.
I believe this effect emerges via hydrated melanin biology. I believe this is how the RPE regenerates itself constantly over human life. 380 nm light signal DHA to become anti-inflammatory and stimulate wound healing via docosanoids and elovanoids. These lipids have to show up before the inflammation of wound healing can begin. This is why diabetic retinopathy looks like a wound that never heals on the ophthalmoscopic exam. IT LOOKS THIS WAY BECAUSE DIABETICS NEVER SEE THIS frequency of LIGHT because of their light choices. It is also why their wounds do not heal and their nerves does not work. It is also why they get peripheral arterial disease. It is all a light story.
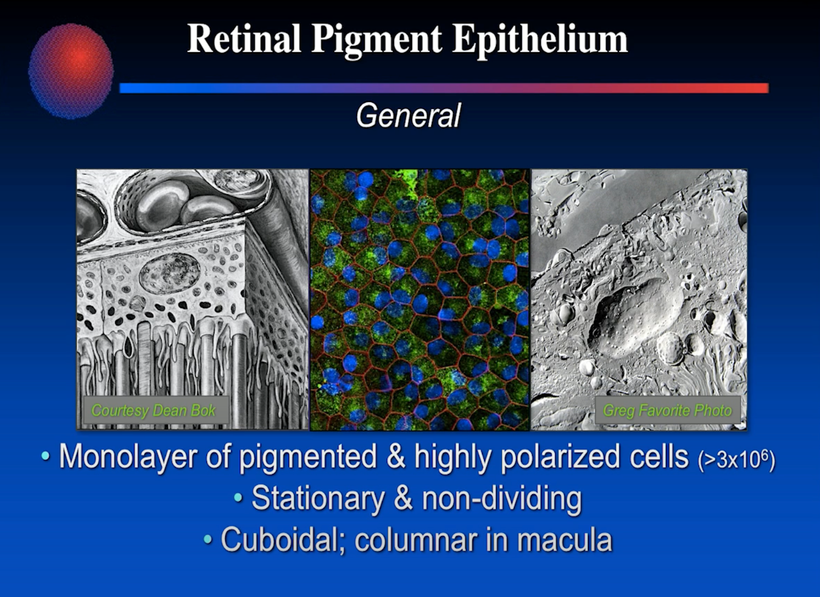
The RPE is the only melanin sheet in the body that ophthalmologists are taught cannot be regenerated in their textbooks. I totally disagree with this centralized viewpoint because of what I have learned in my own clinic. I have restored hundred of RPE’s You can see this belief below used to teach residents at an Ivory Tower in the Western US.
The photo repair mechanism works most efficiently at 380 nm — the same frequency that mTOR operates on and this is the same frequency that the neuropsin receptor senses. Fewer people know it is the exact same frequency that Popp found was scramble most by cancer-causing compounds he used when he first got involved in the field of biophoton research. He realized that chemicals that cause cancer scramble incident light at 380 nm and once the light was emitted the frequency was different.
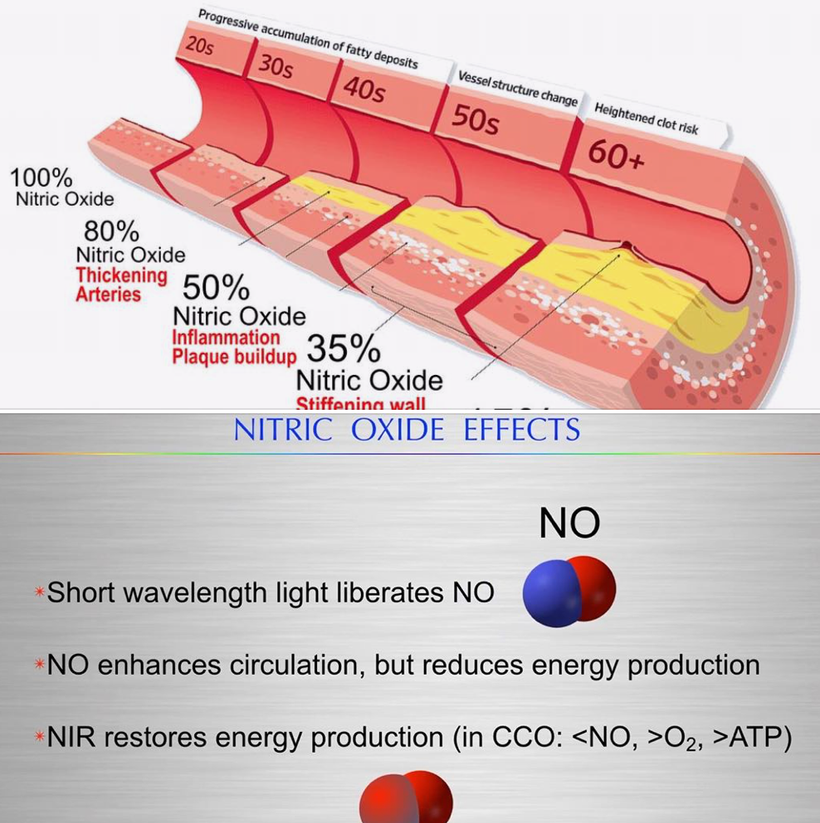
The difference in the light frequency changes the nuclear genome reaction. I also think this light is a key trigger in POMC cleavage (hint). In Popp’s experiments, chemicals that did not scramble the incident light at 380 nm did not cause cancer. Popp never realized that the scrambled light is what turned on the cellular machinery in a chaotic fashion. Without 380nm light, the band of musicians in biochemistry that are creating light emissions around 380 nm is now creating a massive light show that overwhelms all the chromophores inside of us. When this occurs melanin is DEGRADED and DESTROYED because of the overwhelming chaos it causes rapidly. This damage occurs at the speed of light in a cell. PAD and NO levels are the best proxies I know that signal photo repair is seriously defective in my patients.
The one disease I think illustrates this mechanism is glioblastoma multiformans. Every single neurosurgeon who will ever read this blog in the future knows these tumors seem to appear out of nowhere in many cases and none of us can explain it. I think I can because of this mechanism. And I think the literature has clues that I am right. See the slide below about gliomas. If your Vitamin D is low, it is beyond likely so will your UV-A light exposure.
In fact, nitric oxide levels in tissues are a better proxy for understanding whether 380 nm light is stored at the vibrational level in your cells to direct photo repair. This is why my clients all get their nitric oxide levels tested. NO is the best proxy for 380 nm light that a decentralized clinician can test. I believe this is why NO also has to interrupt mitochondrial energy production. To stop destruction of melanin degradation and begin melanin renovation it makes thermodynamic sense that you need to stop energy flow to a damaged tissue before the process changes to regenerate. The stimulus happens with this new “Jacquard card signal” in the cell. My Kruse for Dummies folks will understand this analogy best. Most of my patients have no idea what I am really looking for when they come to see me as a client when I am looking in their eyes, but now you do. It certainly isn’t about their blood pressure. When a man is on viagra or a women had difficulty orgasming, sometimes I do not even need to look into their eyes because I already know. Same thing is true if they are young and infertile. That all links to the same pathway. The leptin melanocortin pathway.

Fritz Popp also never knew that neuropsin was everywhere in mammals, eyes, cornea, and skin. It is also inside our brain at key locations close to melanopsin. Neuropsin is a nonvisual photoreceptor that is attuned to this light frequency. (see slide below) This is why I have been using UV-A light with a spectral density of 380nm with my clients for ten years now. I bet a few of them just fell back in their chairs reading this.
Neuropsin is the afferent loop chromophore protein that works with hydrated melanin sheets inside mammals to direct photo repair. The efferent loop of this light reflex is the production of docosanoids and ELVs from DHA. This is not a recycling or renovation type of healing. This is a full-blown regeneration of tissue from RBCs and UV light that rebuild melanin. This is why Dr. Becker noted mammals do not seem to regenerate as salamanders did via their somites. We do it best where POMC is buried in our cells based and where we are creating UV-A light internally. We regenerate from within at a subcellular level using non-linear optics!
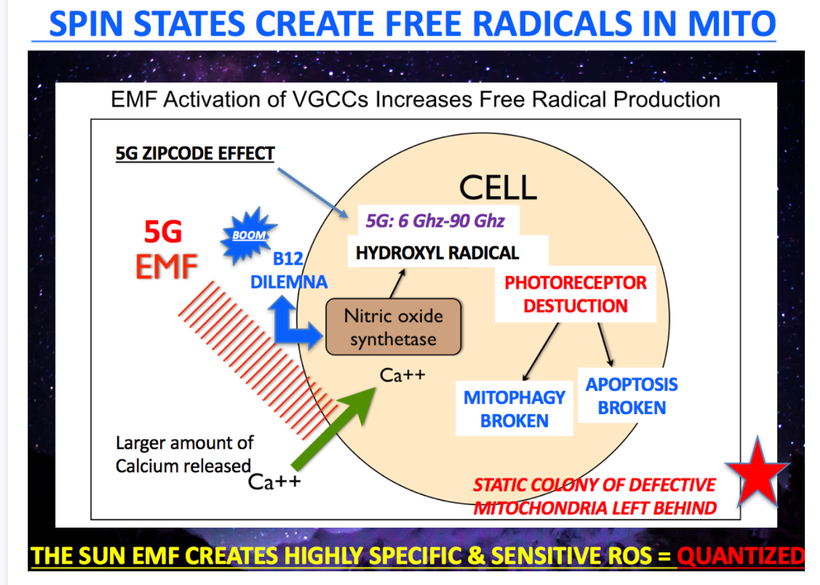
Blue light and nnEMF ruin the dissipative structuring in cells.
The mammalian cell is built to be a dissipative structure because it controls its atoms well. Semiconduction is the ultimate controller of the flow of electrons in a system. This is why Nature uses it as her template. This atomic order allows us mammals to capture energy in some format and store it. Tyrosine allows us to capture sunlight via melanin. When we become able to capture light well order returns to our joints, tendons, and ligaments. Order or health emerges directly from chaos. Things get more ordered in a cell as energy continues to be pumped in. Melanin renovation requires solar capture and storage of this energy and information at the vibrational levels in cells. This is essentially what melanin and water are doing for cells. Water, created by mitochondria and powered by visible light gives flexibility to proteins, reduces the energy barrier between reactants and products, and increases the probability of quantum tunneling by a transient compression of the energy barrier of the semiconductors in the musculoskeletal system.

I have posted many times that sunlight is a phenomenal calcium channel blocker. I also have posted many times about how nnEMF alters calcium flows. I have been amazed at how long it has taken anyone to put two and two together. Melanin creation protects us from electromagnetic pollution because of its effects on calcium flows in cells and in mitochondria. Calcium is critical in semiconduction inside the mitochondria.
Note the photoreceptor destruction issues below. Melanin is one of the photoreceptors this slide deals with. So things that protect photoreceptors are all part of the melanin renovation program. Not surprisingly, docosahexaenoic acid (DHA) is a big one for the RPE in the retina. DHA is broken down into docosanoids & elovanoids via hypoxic oxidative damage. THIS DAMAGE IS REQUIRED FOR RENOVATION.

Elovanoids (ELVs) enhance the expression of pro-survival proteins in cells undergoing uncompensated oxidative stress. DHA helps stimulate autophagy to recycle photoreceptors in mammals. We have the most DHA in our tissues as a mammal for a reason; our brain is unique in the mammalian family. We need A lot of DHA because we use more non-visual photoreception to run our nervous system properly. This is why I wrote the Epi-Paleo Rx long ago. You might want to read it again at some point since this blog is putting Windex on your glass eyes. You must understand why this step is critical. Without DHA photo repair of your melanin sheets fails in humans.

We make docosanoids & ELVs in cells that have huge amounts of DHA & melanin. In mammals, this is good news because most of the melanin inside their bodies is adjacent to DHA in their membranes around the perikaryon. Docosahexaenoic acid (DHA, 22:6 n-3) is abundant in the retina and is enzymatically converted into pro-homeostatic docosanoids. The DHA- or eicosapentaenoic acid (EPA)-derived 26 carbon fatty acid is a substrate of elongase ELOVL4, which is expressed in photoreceptor cells and generates very long chain (≥C28) polyunsaturated fatty acids including n-3 (VLC-PUFAs,n-3). Dr. Bazan did all this work right before my residency and this is how I learned about it.

How do we know DHA is protective of photoreceptor autophagy and a huge part of melanin renovation Rx? Because giving mammals chloroquine, a potent inhibitor of mammalian autophagy can turn off the protection of DHA metabolites like the elovanoids. Peer-reviewed papers from many labs (including Dr. Bazan) now have confirmed and concluded that DHA elicits neuroprotection by regulating multiple cell death pathways including enhancement of autophagy and inhibiting apoptosis and necroptosis in mammals.
Lipid mediators such as prostaglandins and leukotrienes play pivotal roles in the initiation of acute inflammation (Samuelsson, 1983), whereas resolvins and protectins promote and stimulate active resolution (Bazan et al., 2010; Serhan et al., 2002; Serhan and Savill, 2005). In excess, prostaglandins and leukotrienes are pro-inflammatory. When the omega 6 pathway is induced to cause inflammation aspirin is the best choice because it does not affect DHA breakdown mediators resolvins, protectins, and maresins which turn off inflammatory cascades to begin regeneration cascades. So the use of aspirin is part of an early melanin renovation program for some clients, but not all. It depends on their N = 1. You’ll see why below.

Below you can see melanin can contain the calcium flows that are uncontrolled in blue lit or nnEMF environments we have created. When calcium varies so does the light spectrum of light our cells release. This is why Fritz Popp found we are most unhealthy when we are releasing light as a eukaryote. We are losing our ability to store energy at the electronic level because melanin and water cannot contain it. It is a sign of wide-band gapped semiconductors failing us.
In the 1970s, Dr. Fritz-Albert Popp and his scientific team at the University of Marburg began researching the fascinating subject of biophotons in eukaryotes. He extended that to all life forums based on his findings. They were eventually able to demonstrate that biophotons, as ultra-weak photon emissions (UPEs), were released by the body’s cells – all of the cells! Most of the light he found was in the UV and IR range.

Initially, for his experiments, Popp chose to work specifically with UV light because of the experiments of a Russian biologist named Alexander Gurwitsch who, while working with onions in 1923, discovered that roots could stimulate a neighboring plant’s roots if the two adjacent plants were in quartz glass pots but not if they were in silicon glass pots. You heard me tell Dr. Huberman that this experiment is the key to understanding biology and human evolution. It certainly is key in understanding photo repair and regeneration of mammals for longevity.
UV light is what translated the POMC gene in mammals. The only difference was that the silicon filtered UV wavelengths of light while the quartz did not. Gurwitsch theorized that onion roots could communicate with each other by ultraviolet light to stimulate mitosis. It appears UV light ROS is key to moving the cell cycle along. This idea implies there must be a break in the cell cycle at some level to induce photo repair. It turns out there is 380nm light. Note in the slide below how at 380 nm ROS from UV light drops like a rock. My Kruse for Dummies attendees will remember what Shannon said about messages and their entropy. What makes a message memorable? THEY HAVE TO BE UNUSUAL TO BE NOTEWORTHY.

Messages have to be unusual to be recognized optically. A sudden drop off of ROS fits that bill. When they are unusual entropy drops in a system. When we heal, we are decreasing entropy in a cell because we are restoring order from chaos.
Coincidentally, this is the frequency that seems to be associated with the catabolic anabolic switch of mTOR, and the creation of docosanoids and elovanoids of DHA breakdown. Does nature engage in coincidences or does she have a plan few see?
VIDEO
Humans are filled with trillions of cells with their associated 100,000s of biochemical reactions and this means quadrillions of light photons are transformed from the matter in those biochemical pathways. This is why a loss of melanin efficiency can create havoc we call disease because we use it to create chemical energy when the biochemical energy pathways are defective for any reason!
This is the essence of the melanin renovation Rx. There is no replacement for the sun ever. If anyone tells you this, you must run as far from their advice as possible.

380 nm light is not always present at all latitudes, hence this is why sunlight is always linked to longevity. The further you are away from the equator the further you are from melanin regeneration. We all just saw this recapitualted in Denis Ranacourt thesis on all cause mortality in COVID. Trust me I did no miss that nugget. I am also sure Nayib Bukele will be happy about this news too at my “Age of Light” presentation on March 14, 2024.
Your body contains literally trillions of cells. In only one second of time, just one of your cells is typically involved in over 100,000 chemical reactions. Now think of ALL the cells in your body undergoing ALL these chemical reactions on a continual basis. As you can see, there is a massive amount of cellular activity taking place in your body at any one time – it’s almost incomprehensible. How can your body coordinate all these biological reactions?
The use of semiconduction allows for this control. One of our genomes creates one part of our wide-band gapped semiconductors. Nucelar DNA controls the protein side. Mitochondrial DNA controls the water portion of these semiconductors. The combination of both is what is the real basis that made complex life possible after the Great Oxygenation event on Earth.
I reject the biochemical view of life, as Gilbert Ling did. Hopefully, you can now see why these new concepts caused quite a stir in the centralized scientific community because they only accepted a more static, linear view of the body’s biochemistry. None of them know this occurs below the level of their understanding and fewer realize a physicist has already proven beyond a shadow of a doubt this is occurring in every living thing below the perception of most scientists. You have to be curious to dig. (see below pic)

Another biophysics lesson above: The Moai statues of Easter Island are a lot like modern biochemistry domination of centralized healthcare. Since the 1930s what has been published in books was all centralized science saw. It was the Moai heads………..
It is not what you know that matters……..it is what you notice that will cause you to dig deeper when your mind is well and thinking in decentralized fashion.
Then the quantum scientists and clinicians decided to look where the centralized rulers of modern truth didn’t and new perspectives manifest. Never forget this lesson. Your perspective frames your beliefs. None of them looked at the absorption spectra of the proteins tied to photo repair. I did and that is why people who follow me win and why those who do not are in Big Pharma prisons.
Intelligence and capability are not enough today. There must also be a passion fueling the actions of doing something beautiful. Devotion to the truth is the hallmark of modern morality; there is no greater, nobler, more heroic form of devotion than the act of a modern person to question the very foundations of all centralized institutions because these people simply assumed the responsibility of critically thinking = Survival of the Wisest >>>> survival of the fittest. Being fit is no longer the best longevity option. Decentralized critical thinking has replaced it.

HAS POPP’S WORK BEEN CONFIRMED BY OTHERS?
A Russian scientist, Sergey Mayburov, observed that eggs from fish and frogs released biophotons that communicated with each other in short, synchronized, quasi-periodic bursts.
His previous experiments showed that fish eggs which had been stored in different locations were able to coordinate their growth and development with each other through the use of specialized biophotons.
This is another example of how the light emitted from cells (“biophotons”) is able to carry sophisticated information that affected other biological systems in the environment. This certainly alters mainstream concepts about how the body’s biological systems truly work. I am not interested in centralized science accepting my work. I want to burn that paradigm to the ground and begin anew. With a new understanding of how we should be treating patients.
WITH MELANIN IN TISSUES COHERENCE BECOMES EASY IN A WARM WET ENVIRONMENT.
Coherence vs. Incoherence
Biophotons created from melanin are characterized by light coherence which is composed of highly structured and organized frequencies of light. Think of an orchestra where all the players are playing instruments with the same rhythm. When the music is synchronized, it is harmonious and pleasant to listen to. That’s coherence.
Now, picture a few orchestra members who are way “off the beat,” playing at different speeds. This discordant music sounds chaotic – not pleasant at all. That’s incoherence.
When melanin is degraded or destroyed or water is missing from the matrix the orchestra cannot play and sounds bad and that is fundamentally what disease is.
The goal of your cells’ continual release of biophotons is to create coherence in your body’s orchestra of all its parts – to build the most harmonious, healthy body.
Melanin seems to work best with biophoton emission. Biophotons consist of light with a high degree of order, in other words, biological “laser” light. Such a light is very quiet (low-noise) and shows an extremely stable intensity, without the fluctuations normally observed in light. Because of their stable field strength, its waves can superpose, and by virtue of this, constructive and destructive interference effects become possible that do not occur in ordinary light.
The biophoton release in humans occurs in the visible and UV part of the electromagnetic spectrum at ultra-low intensities, on the order of 10^-16 – 10^-18 W/cm2.
When I was in medical school we used Lehninger’s book on biochemistry to learn the topic. I remember reading a passage in that book. He himself was a biochemist and became a Nobel Prize winner. He mentions in his textbook that some reactions in the living cell happen quite a lot faster than what corresponds to a 37-degree temperature. The explanation seems to be that the body purposely directs chemical reactions by means of electromagnetic vibrations (biophotons).
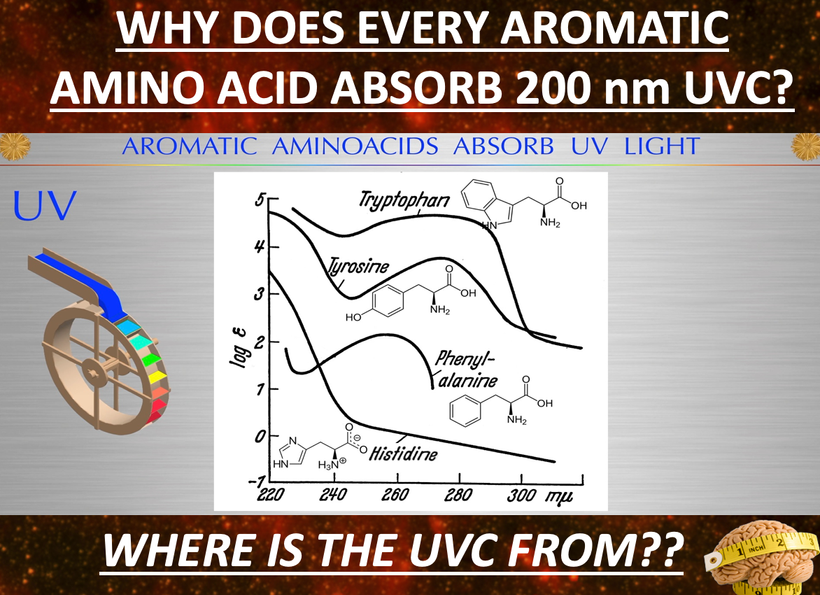
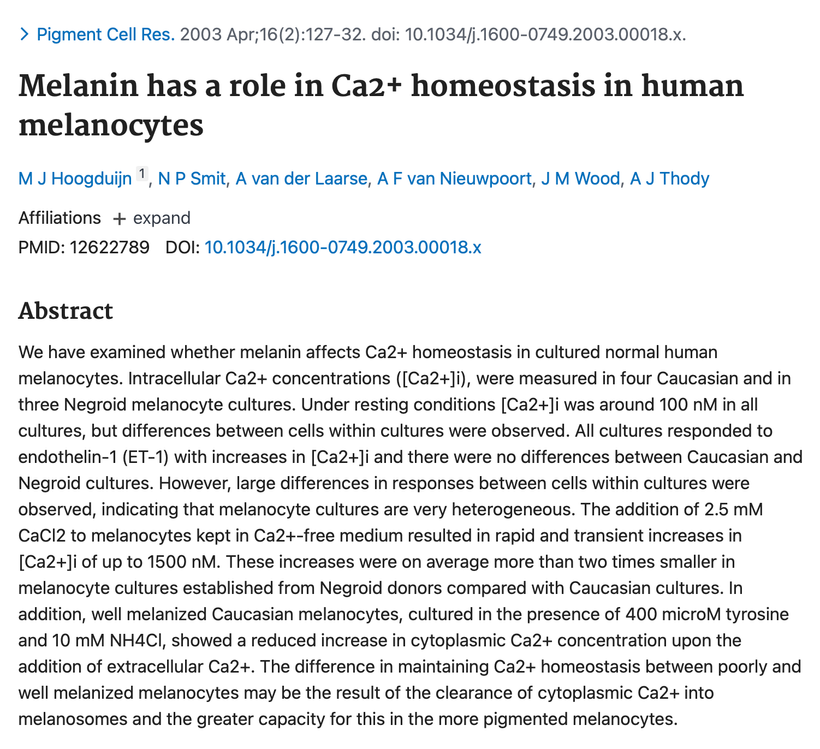
https://pubmed.ncbi.nlm.nih.gov/11277492/ Tyrosine has a massive effect on alpha MSH production. So does tyramine and why things that have it must be avoided during a renovation.
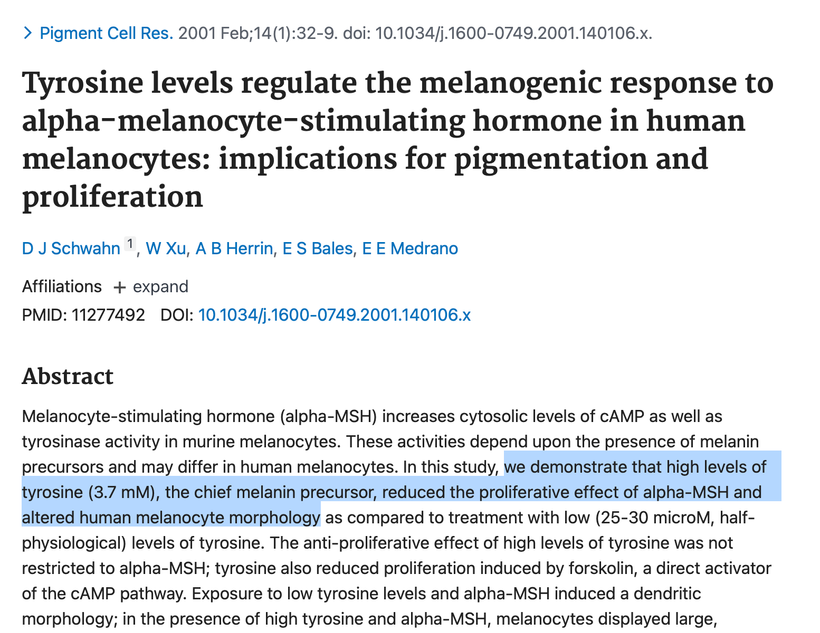
The other aromatic amino acid phenylalanine has the opposite effect. People using protein powders and manufactured food supplements in the body-building community or for ketogenic lifestyles are imbibing massive levels of phenylalanine without knowing it. They believe they are avoiding sugar and substituting an amino acid sweetener and there are no consequences of this decision. This is wrong. Many of them find after training they cannot tan well so they use melotan products to darken their skin. They have no idea the artificial sweeteners in the protein powders are doing them in. After aspartame intake, plasma phenylalanine level is increased, thus influencing neurotransmitter concentration in the brain, and not in a good way. High levels of plasma Phenylalanine actually inhibit tyrosine and tryptophan hydroxylase, the latter of which is necessary for melanin, melatonin, and catecholamine synthesis. Decreasing all three of these by using artificial foods has massive collateral effects on health and can lead to many disorders and early death. (see below)
This is why diet soda makes you FATTER. You are degrading melanin. In fact all diabetic and fat loss diet aids keep you fat by design. Yes, I went there.
https://www.mdpi.com/1422-0067/19/3/746# plants
Dr. Peter Gariaev, a Russian scientist who created the GDV camera, placed some human DNA inside a tiny quartz container and then zapped it with a mild laser (which emits a genuine coherent form of light). The DNA acted like a sponge that absorbed the laser’s light frequencies, storing them for up to 30 days inside an unusual cork-screw-shaped spiral. Even more interesting is that Dr. Gariaev found that after he removed the quartz vial with the DNA, his machines still detected photons of light that were spiraling in the same cork-screw spiral as if the DNA were still present. This “phantom” spiral seemed to persist for another 30 days.
Dr. Gariaev’s work suggests that as-yet-undiscovered forces act to hold the DNA light emissions in place. Does your own cellular DNA exchange information (in the form of light frequencies) with the external energy field in which it is located? In essence, this appears to be the case. This makes sense when you understand Dr. Luc Montagnier’s experiments I mentioned in the Huberman/Rubin podcast. The biophoton effect of our bodies is like a giant energy antenna that is able to constantly send and receive signals by interacting with the external biofield around it.
WHAT MIGHT HELP MELANIN RENOVATIONS DOWN THE ROAD?
https://www.mdpi.com/1422-0067/19/10/3211 PEMF with Helmhotz coils might be helpful but no power density studies in humans have shown good results like we see in Zebrafish. More work needs to be done on this possibility as of 2024. Microphthalmia-associated transcription factor (MITF) is the master gene involved in pigmentation and it works in concert with mitogen-activated protein kinases (MAPK) and peptides mtDNA liberate. We do not know enough yet to recommend this.
We do know however the use of PEMF is plausible because of Dr. Frohlich.
Dr. Herbert Frohlich (1905-1991), who I wrote about on my blogs 15 years ago, who was nominated for the Nobel Prize in Physics, believed that radiation and oscillating waves were responsible for synchronizing the body’s cell divisions and sending chromosomal instructions to various parts of the body. This is reminiscent of the ephaptic neuron transmissions I mentioned in the Huberman/Rubin Tetragrammaton podcast.
Frohlich found in his physics lab that a collective body vibration or frequency was responsible for getting the body’s proteins to cooperate with each other and to carry out the instructions of cellular DNA. He called these “Frohlich frequencies.”
We now know this as molecular resonance theory. Turin used these ideas to reject the lock and key mechanism that was given a Nobel Prize.
Frohlich’s research showed that once energy reached a certain threshold, cells began to vibrate in unison until they reached a high level of coherence. Once cells reached this state of coherence, they could take on certain remarkable qualities of quantum mechanics, including non-locality — or the ability to interact with the external field (the environment) they were located.
What does it all mean with respect to the Melanin Renovation Rx?
To brilliantly feed our personal “body of light,” we need a copious amount of sun. The sun is irreplaceable in this renovation. POMC needs abundant UV stimulus to make the system all work with fidelity. The stronger the better. Your job is to refill every nook and cranny of your cells at the electronic level with energy. Sunlight is the best way because our system is adapted best to full spectrum light.
https://www.mdpi.com/1422-0067/19/10/3149 (avoid ginger because it blocks melanin too due to its anti-inflammatory effects on COX and LOX enzymes)
AVOID ALL ASPARTAME PRODUCTS COMPLETELY
You want to avoid all products with aspartame as well during melanin renovation.
Aspartame consists of two amino acids (L-phenylalanine and L-aspartic acid). It is hydrolyzed and absorbed in the gastrointestinal tract (GI) through the action of esterase and peptidases. Digestion releases methanol (10%), aspartic acid (40%) and phenylalanine (50%)
Phenylalanine (Phy) is needed to synthesize tyrosine, dopamine, noradrenaline, and adrenaline. However, Phy demonstrates a strong affinity to large neutral amino acid (LNAA) carrier systems, thus competitively inhibiting other amino acids which rely on this carrier system (tyrosine, tryptophan) and preventing them from crossing the BBB. Tyrosine is how melanin is made best in humans. Tryptophan creates melatonin. These things are both hindered by exogenous phenylalanine use.
Who eats more aspartame than anyone? Bodybuilders who compete and eat food products and those who try to lose weight using bro science.
Many bodybuilders have made the mistake of believing this bro science from gyms. Drink a Diet Coke after tanning to lock in the tan before a meet.
Aspartame -> Phenylalanine -> L-DOPA -> L-DOPA quinone -> melanin.
This bro science is 100% false. Drinking or eating anything with aspartame in it is the best way to lose a tan because it makes the cell hypoxic. Second best is chronic airplane travel. Try to avoid this when you are trying to rebuild melanin.
Aspartame stimulates melanin degradation. Aspartame intake also results in elevated H2O2 levels and methanol levels, placing added oxidative stress on cells, and both act to lower melanin levels. H2O2 bleaches melanin and methanol is metabolized to formic acid and formaldehyde which ruins protein semiconduction. Aspartame has another nasty side effect on melanin. It also increases cortisol, via ACTH signaling further mimicking blue light stress and this decreases alpha MSH functioning. It is a compound that atrophies the skin and gut microbiome. Its use in bodybuilders is why many of them have short lives. Use of aspartame is a neurotoxin and stimulates many of the pro-inflammatory pathways that increase inflammation. Andy Haman died from aggregation of platelets for example. For example, methanol is an aspartame metabolite and it causes increased levels of free radicals resulting in damage to the cell membrane, caused by peroxidation of fatty acid in the phospholipids leading to a pro-inflammatory cascade of cytokines that lead to clotting. These cytokines, in turn, damage cellular components such as melanin, platelets, proteins, and nucleic acid lesions, as well as gene damage and repair resulting in apoptosis or necrosis. Methanol inhibits phosphorylation in all cell lines.
Note the meatheads online think these three guys below are FIT and know more than me because I do not look like them. The question for you after this blog is, were they WISE even though you thought their facades were better than mine? Do you want a manufactured facade or an epic brain to live out your life?

Recall that mitochondrial uses calcium atoms for signaling (pic is above in blog). Aspartame activates various calcium channels in neurons resulting in cell death. Calcium influx activates calcium-calmodulin-dependent protein kinase II (CaMKII). CaMKII induces the cAMP response element-binding protein (CREB-P) pathway. The top picture in this blog shows you this pathway.
In addition, elevated free radical levels decrease enzyme activity in the liver in these people. This also degrades melanin because it degrades glutathione. Aspartame directly alters glutathione peroxidase and glutathione reductase activities.
Aspartame also has aspartic acid in it. Aspartate is an excitotoxic substance that destroys melanin. Persistent aspartame intake also results in a decrease in hippocampal acetylcholinesterase activity. Lowering ACh fosters beta-sheet protein folding and this destroys the hippocampus. All these mentioned factors affect learning skills and memory. They are also seen in Type 2 diabetics and in Alzheimer’s disease. Many long-term bodybuilders destroy their sleep and brains by using these products while working out in a blue-lit gym and no one sees how this creates early-onset disease by melanin destruction.
Mitochondrial oxidative stress leads to apoptosis of adrenal and brain cells. Long-term administration of aspartame has been found to result in degenerative changes in protein folding. Bodybuilders have a propensity to lose their minds. Combined use of aspartame in their supplements, anabolic steroids, and melotan products all act to cause active neurodegeneration of the brain.
Aspartame has been found to be amyloidogenic, being able to spontaneously assemble into an amyloid-like nanostructure, with more intensive aggregation observed at higher aspartame concentrations. The aggregation is characterized by the formation of β-like structures. Moreover, existing aspartame fibrils can induce the formation of amyloid-like structures from other molecules such as proteins and peptides. The structure formed by aspartame can initiate β-sheet aggregation in the Aβ1-40 peptide. This process leads to the formation of Aβ-amyloid fibrils, which are associated with Alzheimer’s disease.
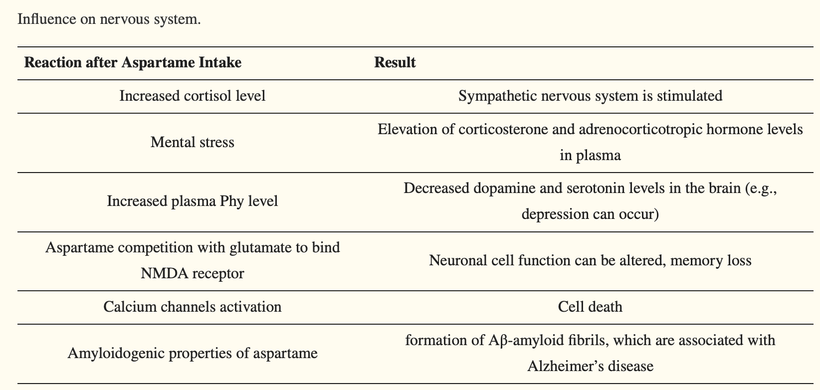
This results in damage to the brain and peripheral nervous system. This includes the sciatic nerves, including demyelination, disruption, and splitting of myelin lamellae, lamellar structure deformation, and myelin loop formation, as well as irregular thickening of myelin sheaths. In addition, other, less frequent axonal changes can be observed: axons can be shrunk, compressed, and distorted, their mitochondria can be swollen, as well as RER dilatation and vacuolation of Schwann cell cytoplasm. Aspartame also appears to have a negative influence on the cerebral and cerebellar cortex. Many bodybuilders have Rhomberg sign just from the combined use of aspartame and melotan products.
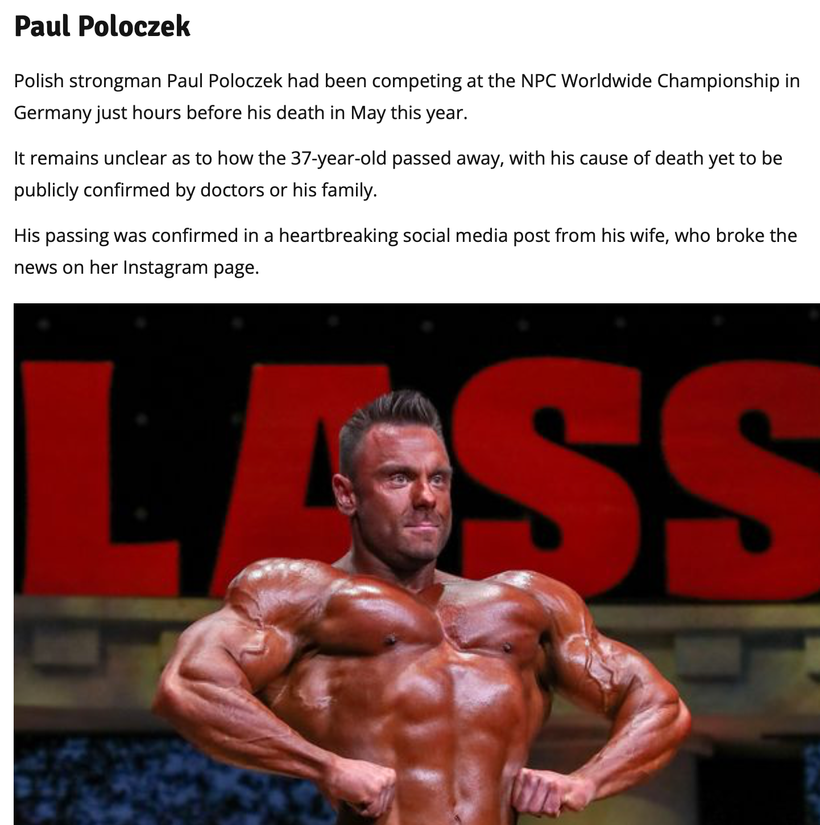
Paul Poloczek was a known heavier user of melotan products and aspartame in his diet to limit the use of glucose while fueling a rise in cortisol due to his blue lit gym addictions. Because aspartame intake also results in elevated H2O2 levels, it places added oxidative stress on cells, This has been confirmed in studies recording elevated nitric oxide and lipid peroxidation levels after a 90-day diet with aspartame. Cite 1 is below.
Many people blame other things on skin and microbiome issues. There is no doubt they are multifactorial, but blue light, fake food sweeteners, and blue light works outs all destroy the skin and gut microbiome. When you add in anabolic steroids it is no shock that the skin facade shows the melanin damage happening below endogenously. Go back above and see what I said about leptin signaling and IL-8 in the skin. The picture below SHOWS IT TO YOU.
Many will look at the physique and not observe the real problem unfolding before their own eyes. I always point it out. So when people tell you fitness leads to survival why do you still believe it? In our modern world, it is no longer true. It is another half-lie that needs to be dissected via Nature’s laws.

^^^This skin here is due to a massive dietary influx of aspartame in fake foods in combination with light stress and PEDs. How? Aspartame can induce contact dermatitis, manifested by skin inflammation, and this has been attributed to an accumulation of formaldehyde in the skin, which is a known metabolite of aspartame.
However, daily aspartame intake must be huge to induce formaldehyde accumulation. The amount of protein powder and liquid supplements people like this guy above takes causes the skin atrophy effect. It also causes a loss of the microbiome in his skin to deal with bacteria. He does not know that his blood-brain barrier and gut barriers remain open to toxins constantly as he eats this way under the blue light while he remains addicted to his tech devices.
The picture above = what diabetic retinopathy looks like on my retinal exams too.
As melanin is degraded you will see red splotches all over the skin as vessels come to the area of inflammation. Such effects have been observed in both adults and children. When sunlight affects the skin properly these effects can be mitigated. Imagine that, sunlight has reduced the inflammation on his neck where melanin is and it shows up with a vengeance where his shirt blocked the sunlight. Are you seeing a trend yet?

AVOID NSAIDs: THEY ALSO AFFECT MELANIN BIOLOGY
Tyrosinase is a copper-containing enzyme, responsible for the formation of the melanin of skin, hair, and eye in mammals. In addition, tyrosinase is found in animals, plants, fungi, and microorganisms. NSAIDs block tyrosinase just like suncreams do (cite #2).
Non-steroidal anti-inflammatory drugs (NSAIDs) are known to act by directly suppressing the activity of cyclooxygenase (COX), the key enzyme catalyzing the biosynthesis of prostaglandins, which induce inflammation. Therefore, NSAIDs are generally used to treat pain, inflammation, and fever. Moreover, researchers have now reported that NSAIDs are able to prevent the development of cancer in the colon, breast, stomach, and lungs. These biological effects of NSAIDs are the result of their ability to induce apoptosis and cell cycle arrest. This was popularized during the approval and early sale of Bextra and Vioxx for colon polyposis. Both drugs were soon removed from the market for causing cardiac deaths. Since I treated a lot of older Americans with osteoarthritis of the spine I wanted to know what I should look for in those at risk for cardiac disease from the use of these drugs. Many people did not look into the cause of the cardiac abnormalities 20 years ago but I did. I found out that all altered catecholamine synthesis and melanin at some level caused their diseases. I never forgot that.
According to the National Strength and Conditioning Association NSAIDs are among the top 3 drugs used by people who use resistance training in the USA. So, this means people spending time in blue-lit gyms lifting weights are creating massive amounts of inflammation from their workouts and from the blue-lit in their gyms. As the inflammation piles up they reach for NSAIDs to make them feel better. It turns out this is not wise. Not only do NSAIDs not limit pain when your blue light/nnEMF is toxic, but they also block tyrosinase and decrease melanin renovation.
The best anti-inflammatory on Earth is sunlight. The second best one is aspirin but it still blocks melanin synthesis. This is why it is second best. Why? ASA does not have the same efficiency as NSAIDS on tyrosinase or on DHA resolvins and elovanoids (cite #3).

Make no mistake about it though, ASA still blocks melanin production in humans as the picture below shows. Microphthalmia-associated transcription factor (Mitf) is a critical factor in melanin synthesis. I have found 380 nm light to be the best stimulator for this gene. Once activated, Mitf promotes gene transcription by binding the promoter region of the tyrosinase gene. Interestingly, aspirin inhibited the Mitf gene at the transcriptional level. This leaves people with atrophic skin who cannot turn on the production of melanin. Because there is still a suppression of the Mitf gene by aspirin, we now know its use depresses the tyrosinase gene and the subsequent inhibition of melanogenesis. This should immediately get many centralized physicians to begin to question how algorithms in stroke and in STEMI are being used today in ED all across the globe pushed by the AMA (pic above). Does it make any sense when you see things from this perspective?
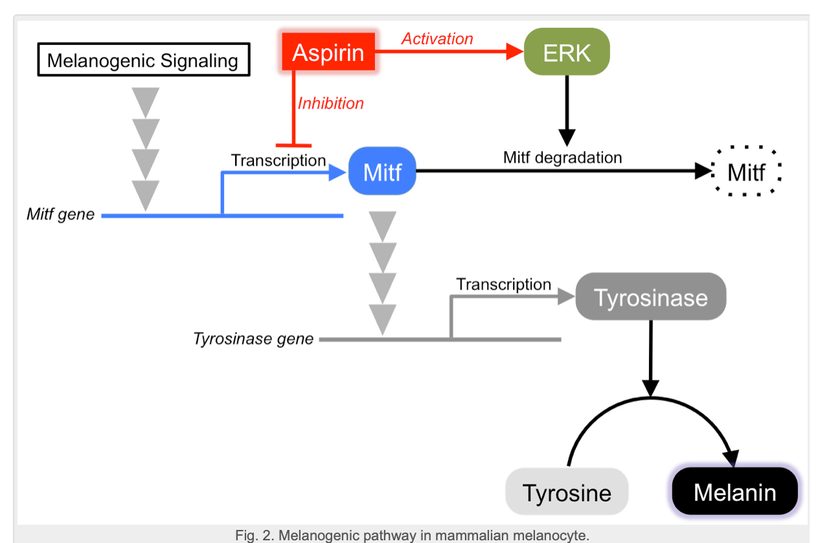
This is why I favor SUN as the BEST anti-inflammatory on Earth for all diseases. If you are still hurt from any disease after a sun tan it means you need more sun not less. Pain thresholds are linked to solar energies stored in cells. This is why drug addicts all have low Vitamin D levels when pain specialists look. Sadly few do. It explains why most musicians use too many drugs too. They are shift workers who rarely see the sun while using electrified instruments under fake light.
It means the amount of inflammation in your body is a lot higher than you can imagine. Light stress in modern life is the main driver of most of these inflammatory cascades in my opinion. It is blatantly obvious to anyone who looks deeper than a biochemistry book printed by Big Pharma grants and centralized labs paid to publish rubbish.

SUMMARY
After decades of layered misinformation (updated and published every 5 years starting in 1980 til the present) by the US government (USDA and HHS) imbedded in the USDA Food Guidelines and summarily adopted and supported by all national health organizations, I came to the realization in medical shool I had no trust in our government or any national health organization. This set the stage for my career and why I think I figured this chronic disease issue out.
“Science” is the word Master or Public Health experts like to throw around on social media but the truth of the matter is they have used for 50 years to abuse the health of the public and no one in media has held them accountable and no Constitution protects the public from these people on the globe as of today. Did COVID-19 response foster public health or public harm? Was the “cure” worse than the disease? The after market data is a clear now.
For some of us we thought it was clear in 2019 and 2020 but we were censored. Words are empty if you don’t act upon them. Action requires purpose. You shouldn’t mistake activity with achievement. To act is to apply. to change, to transform. To assess the quality of thoughts of people, I don’t listen to their words any longer in public health, but I do watch their actions. And then I come up with a plan for my tribe. Then I teach them that plan.
Now is the time for error correction for world governments. Medical tyranny has to stop and there is only one way to put a decentralized feedback loop in to healthcare to stop it. On March 16, 2024 come on down to El Salvador and make sure you are wearing all white if you attend and see what this decentralized plan is all about. The health summit is being put on by the Palestra Society and it will be called the “Age of Light”.
Now you can see why we want you all in white……………
You’ll find out how decentralized public health is returned to the people once and for all. Start at the beginning and question everything: lockdowns, asymptomatic transmission, mask mandates, claims about immunity/variants, and all the bullshit treatments pushed by centralized government mandates and toss them in the trash. We shall be talking all about the tragic comedy of errors that allopathic medicine has been engaged in and start talking about the decentralized solutions we have at our disposal. Follow all these people below.
@nayibbukele
@twocitizenships
@PalestraSociety
Change is coming to the world regardless if they are ready for it or not.
Skeptics and critics who lack wisdom often fall prey to their own expediency. These hungry critics are devoid of understanding. This is why the critic is always in a hurry to sentence those who have skin in the right game. They push other small minds to hang “a sentence sign” on these people who threaten their positions in society. They do this so their followers can try to lynch someone who believes something they cannot fathom is true. In health decision making, when practicality and morality are polarized and you must choose a side. You must do what you think is correct, rather than what you think is practical if you are ever to succeed. When ignorant people don’t understand a mitochondriac’s behavior—who cares what they think?

This is about survival of the wisest now, and not survival of the fittest. Their request that we must only do what the ignorant understand is their attempt to dictate and control narratives to us. It is immaterial to the ignorant who is right or wrong in any topic these days on social networks, just so long as their meme wins crowdsourcing on social media. If this is being “asocial” or “irrational” to the ignorant’s eyes, so be it. Mostly they resent a mitochondriac’s freedom and our courage to think for ourselves. Mitochondriacs owe nobody an explanation or an accounting of facts, especially the ignorant. I let them sink under the wieght of their own Dunning Kruger effect and watch from afar as evolution weeds them out based upon their beliefs. My position is we need to make the Twitter life of these people a total misery by highlighting his gaslighting of the people who were wise to avoid the opinion of centralized medicine and pseudoscience of Fauci, Collins, Daszak, Gates, DoD, Trump, Biden, and Trudeau. I hope my Twitter followers teach these ass clowns a lesson in 2024. They deserves everything they get in my view.
THE BOTTOM LINE: If you are told not to question it by a government, it’s not science, it’s control & tyranny tied to a power grab. Simple end of report.
Heresy has classically been considered dangerous to the indoctrinated “believers”. Heresy isn’t really dangerous; it’s progress for the innovators because they can use their minds and imaginations to observe things that are hard to observe. What we observe is not nature in itself but nature exposed to our method of questioning. If we do not have the tools to see what nature is up to we can never observe her methods, but it does not mean this cannot occur. The laws of classical physics forbade Einstein from innovating prior to 1905. It didn’t seem to get in his way, did it? Pay attention to nature and nothing in centralized medicine will get in your way either when you’re looking for answers to your illness today. The last few podcasts I did I was warming up for this blog. I was warming up for what I am going to bring to the world in 2024.
Today, corporations and NGOs are using their money to make sure many of these observations remain outside of the literature. Realize that is why they try to control the methodologies in the research they FUND. Game, Set, Match. This is how centralized PEER operates to keep the public sick.

I just gave you my life’s work in a five dollar blog. I made it cheap so this would go VIRAL. Take several weeks to digest it. You won’t have another blog for a while because I DEMAND YOU READ IT AND ASSIMILATE IT.
Print this blog up and hand it to every skeptic you know. Gift it to your centralized doctor and tell him it is time to LEVER UP THEIR KNOWLEDGE. It is time to change the world. On March 16, 2024 just watch me do what centralized medicine, DoD, HHS, Big Pharma, tried to stop from me doing with that TED talk. It is game on now. I’ve got an Ark to build.

CITES:
https://www.mdpi.com/2073-4409/10/6/1548
Ashok I., Wankhar D., Wankhar W., Sheeladevi R. Neurobehavioral changes and activation of neurodegenerative apoptosis on long-term consumption of aspartame in the rat brain. J. Nutr. Intermed. Metab. 2015;2doi: 10.1016/j.jnim.2015.09.001.
https://www.researchgate.net/profile/Kazuomi-Sato-2/publication/47795280_Depigmenting_mechanism_of_NSAIDs_on_B16F1_melanoma_cells/links/59ade367a6fdcce55a416edd/Depigmenting-mechanism-of-NSAIDs-on-B16F1-melanoma-cells.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3131604/
https://atlasofscience.org/how-does-aspirin-inhibit-melanin-synthesis/
https://www.linkedin.com/pulse/pushing-cancelled-researcher-finish-line-jack-kruse/
https://atrium.lib.uoguelph.ca/xmlui/handle/10214/14294
Longevity podcast: https://www.youtube.com/watch?v=0tqseGnkbhM
DECENTRALIZED SCIENCE AND MEDICINE PODCAST: https://www.youtube.com/watch?v=VO4JwdXuXXs&t=5s

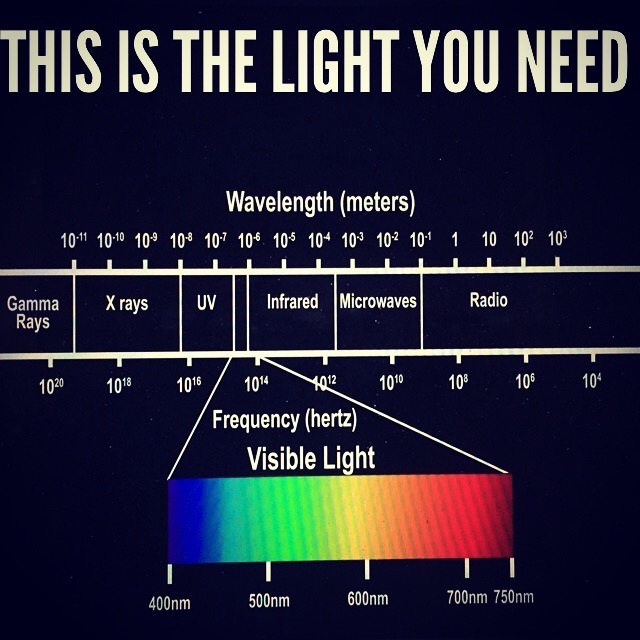
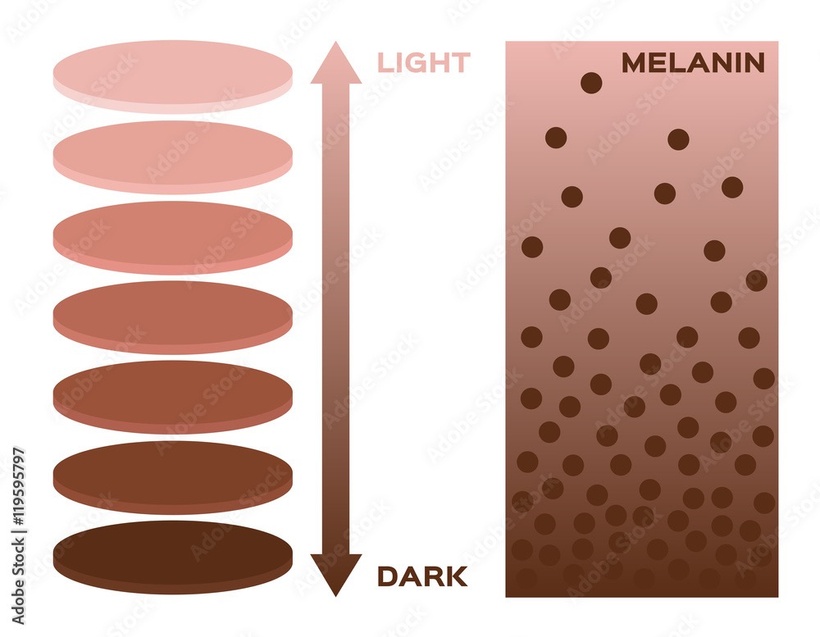



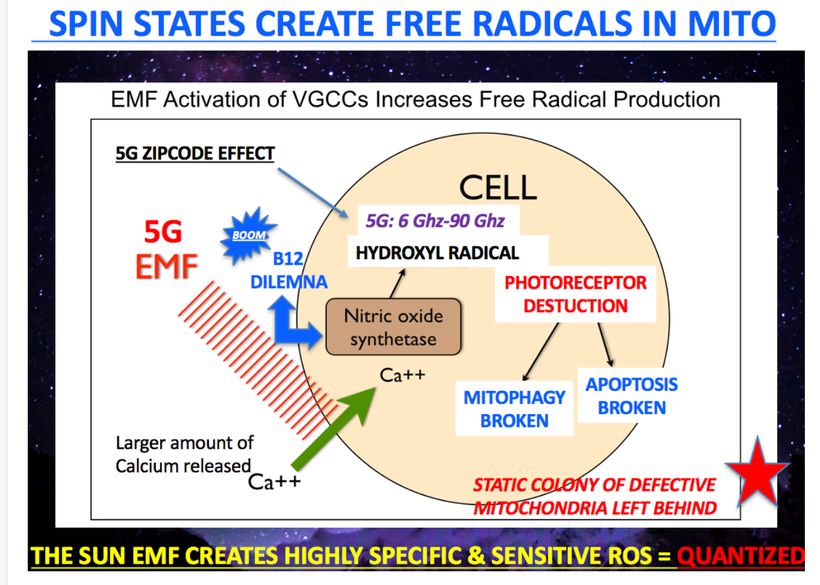




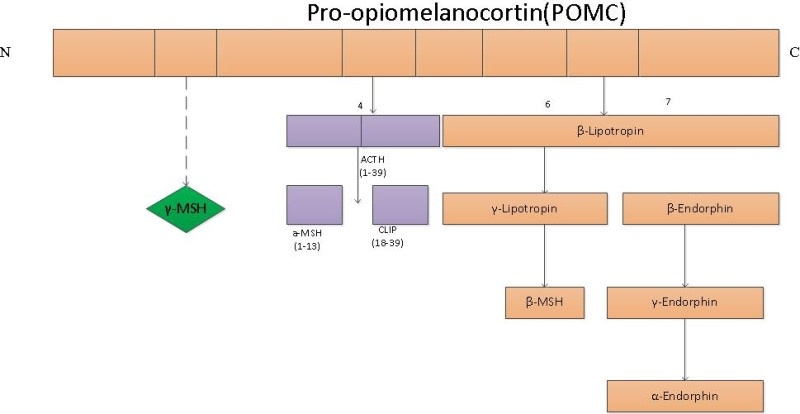


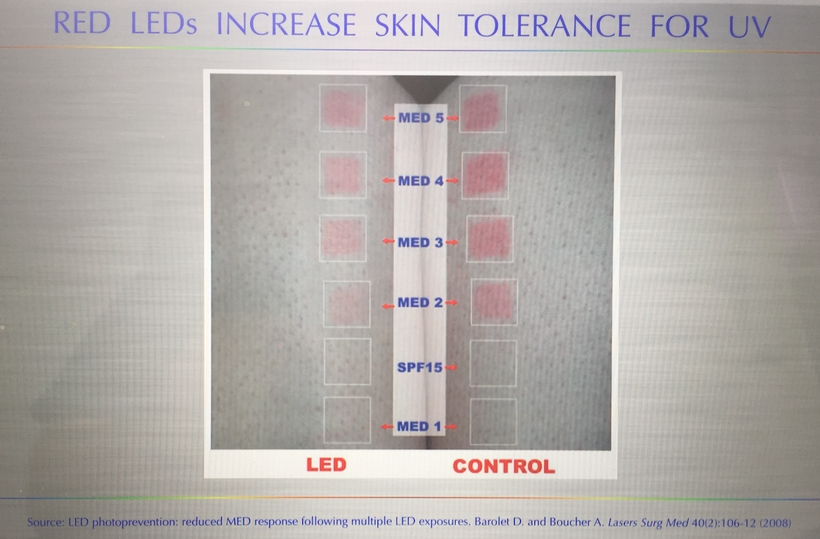








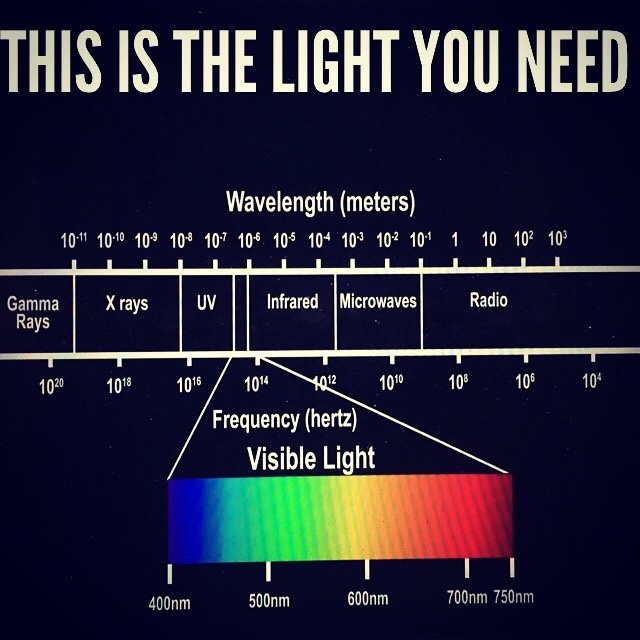














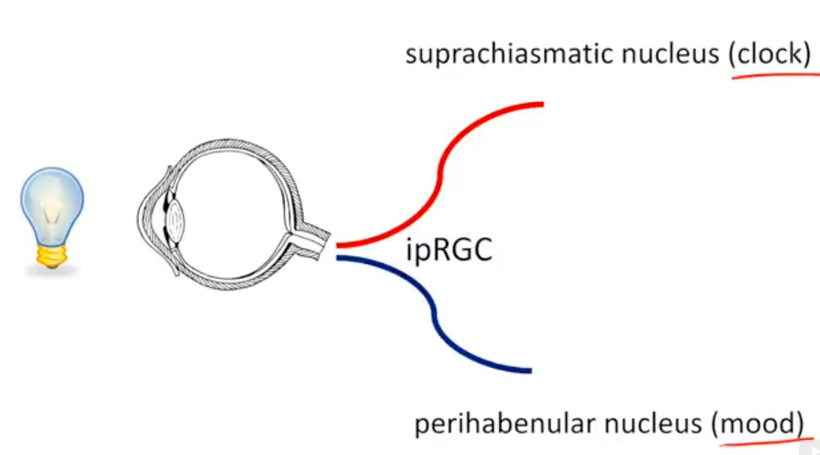
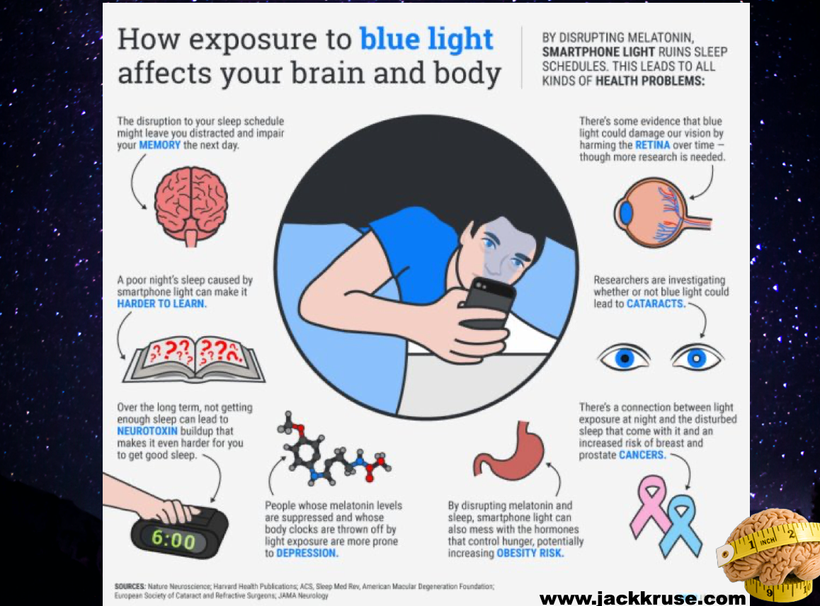

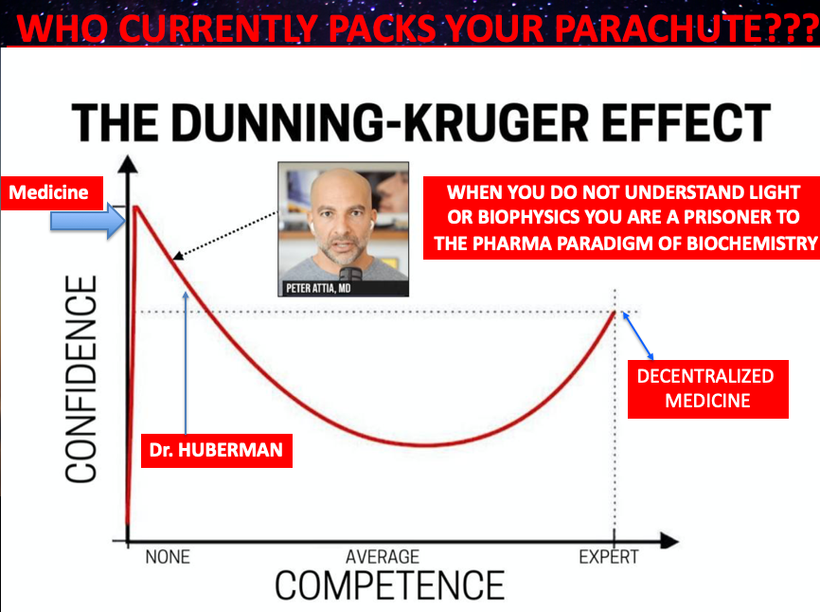
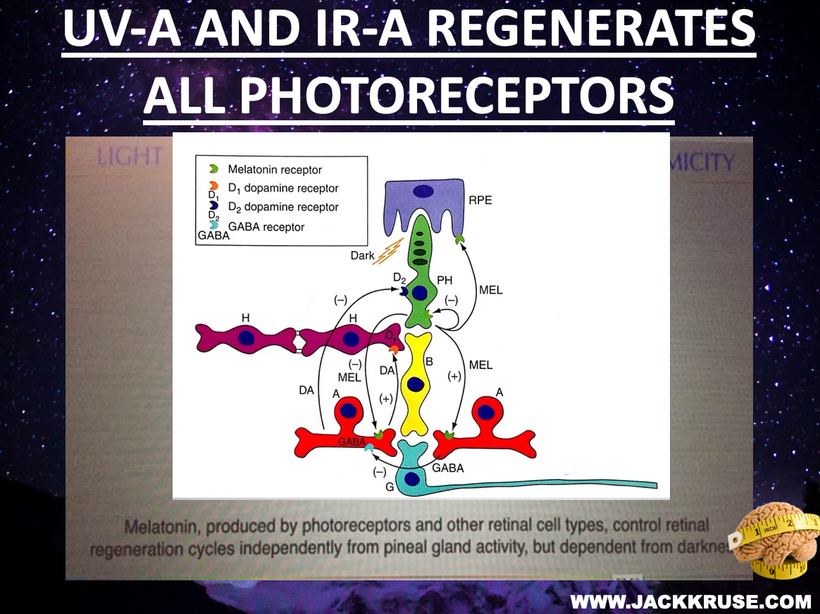
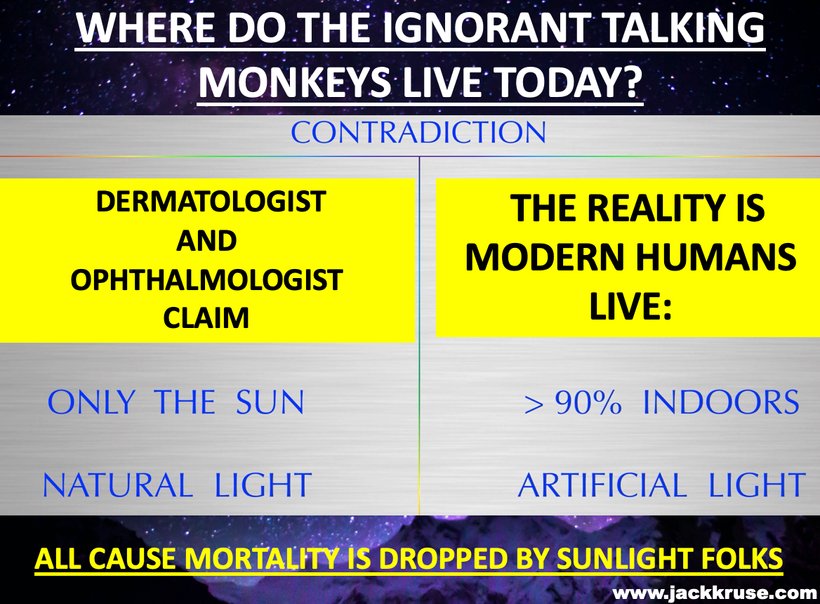




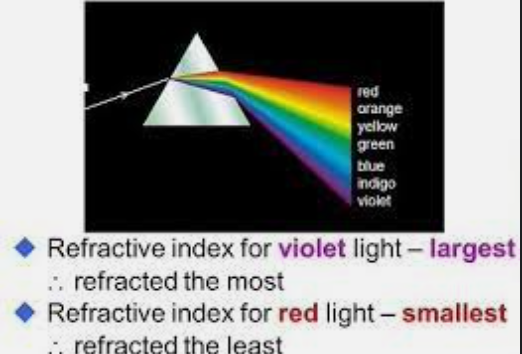







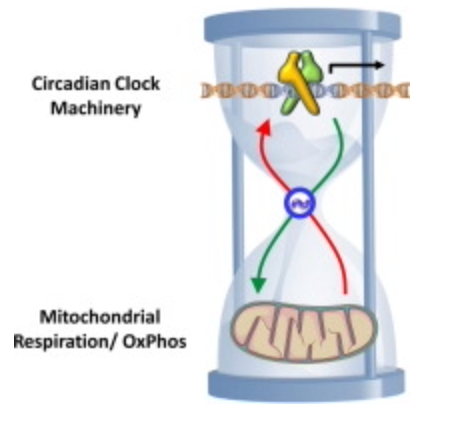
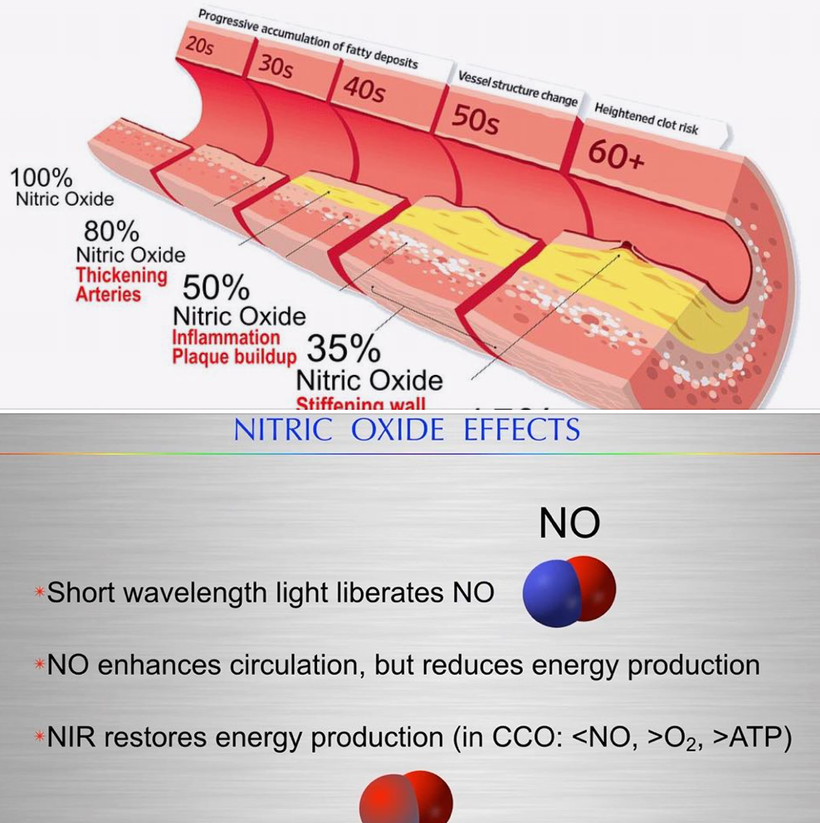
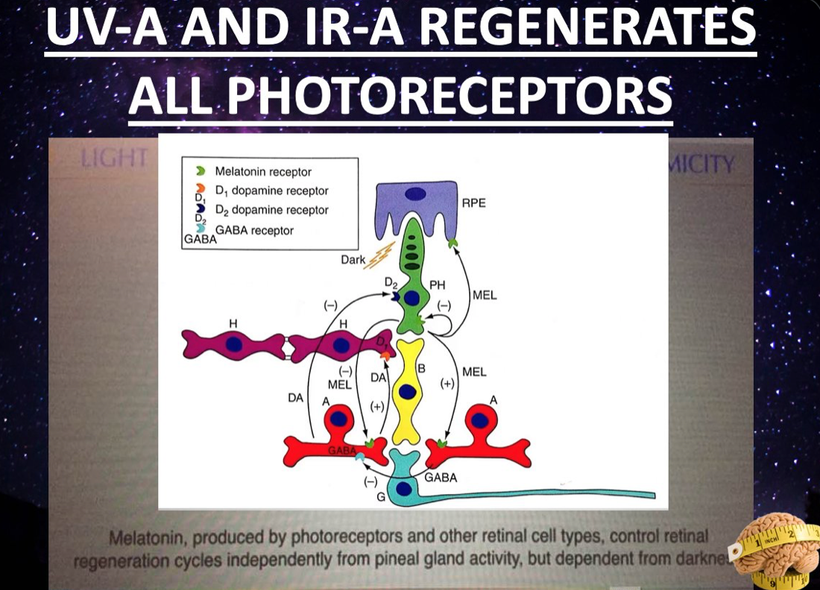






























 IL-1b
IL-1b