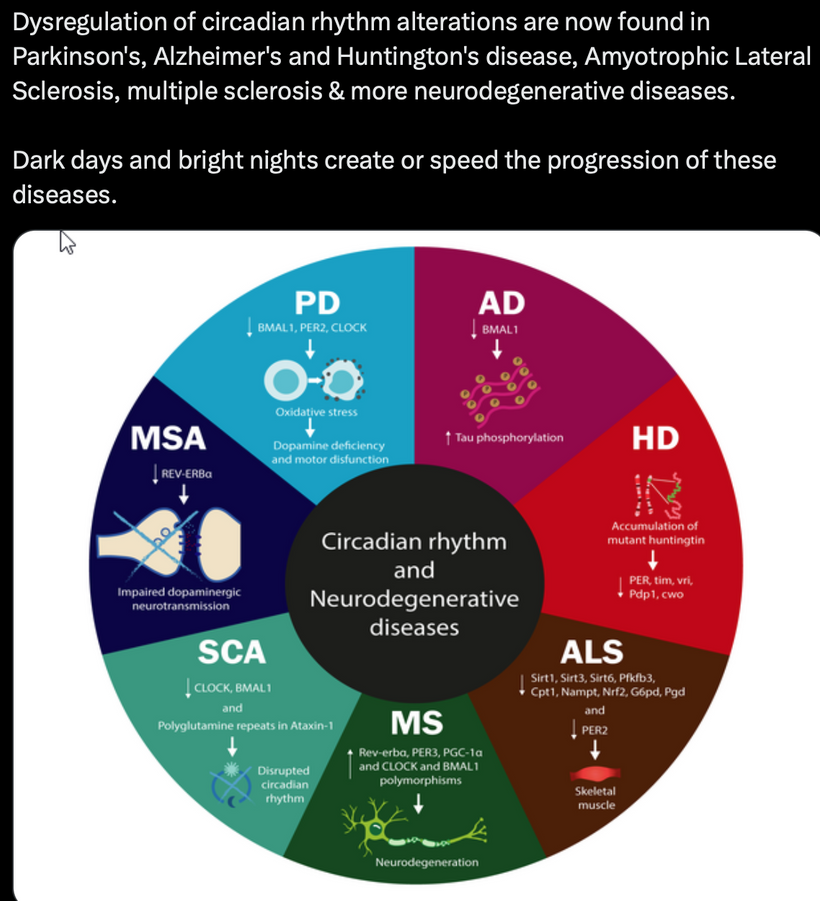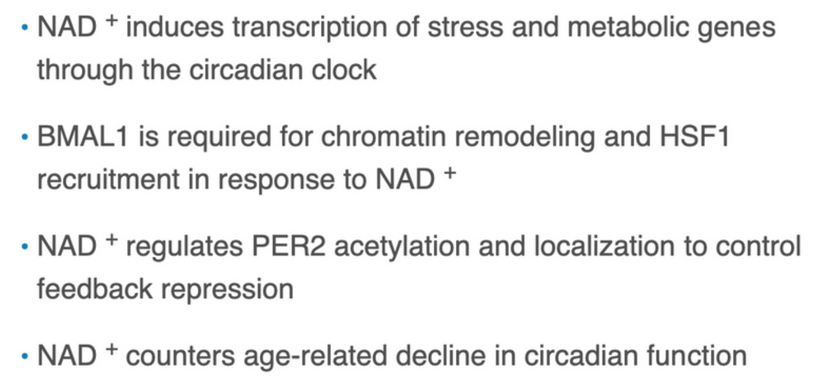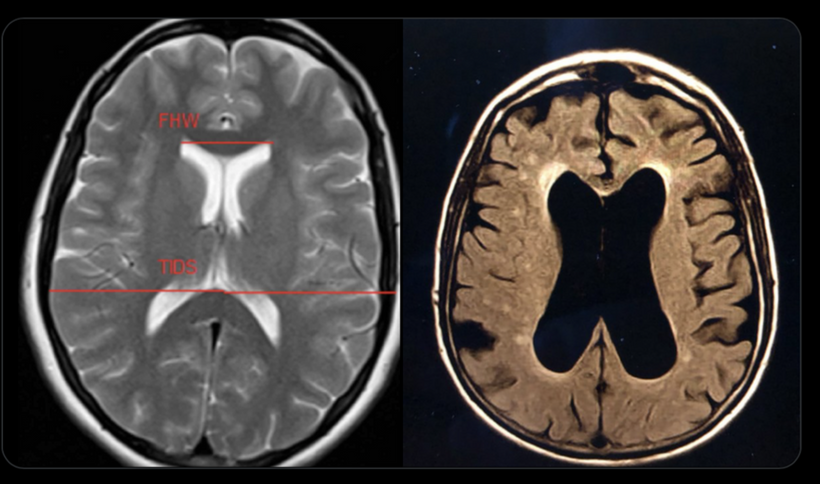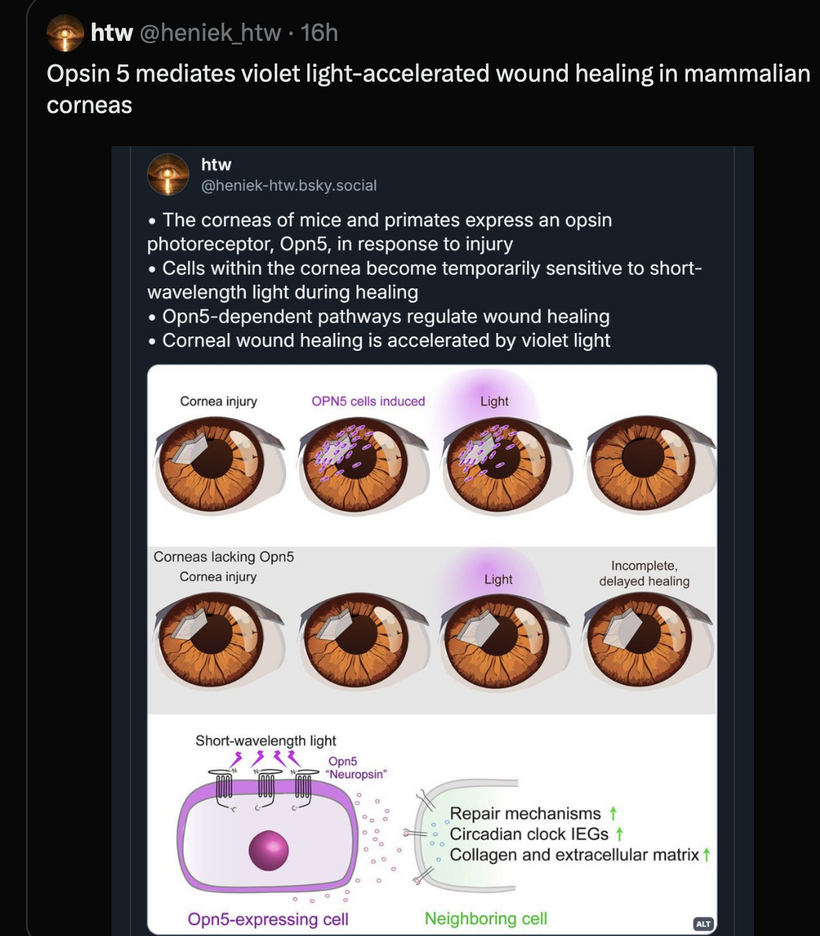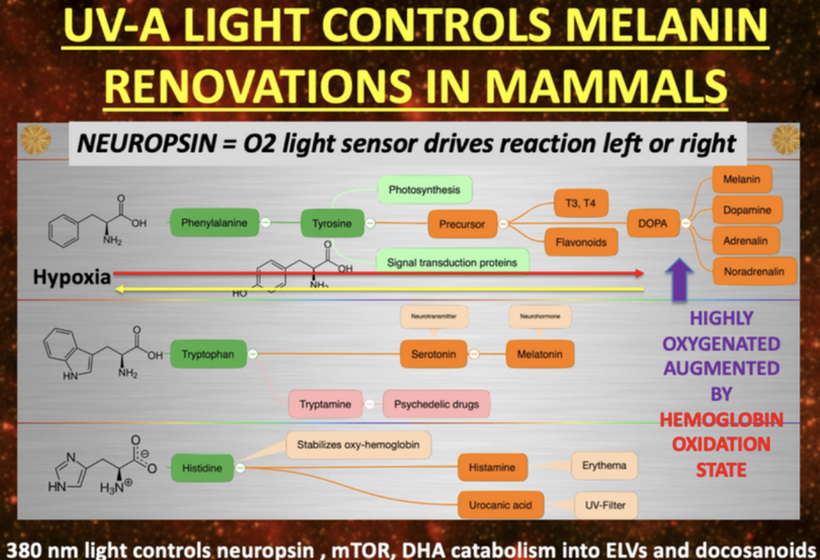
This blog is about why the SUN is TINA for healing of all diseases.
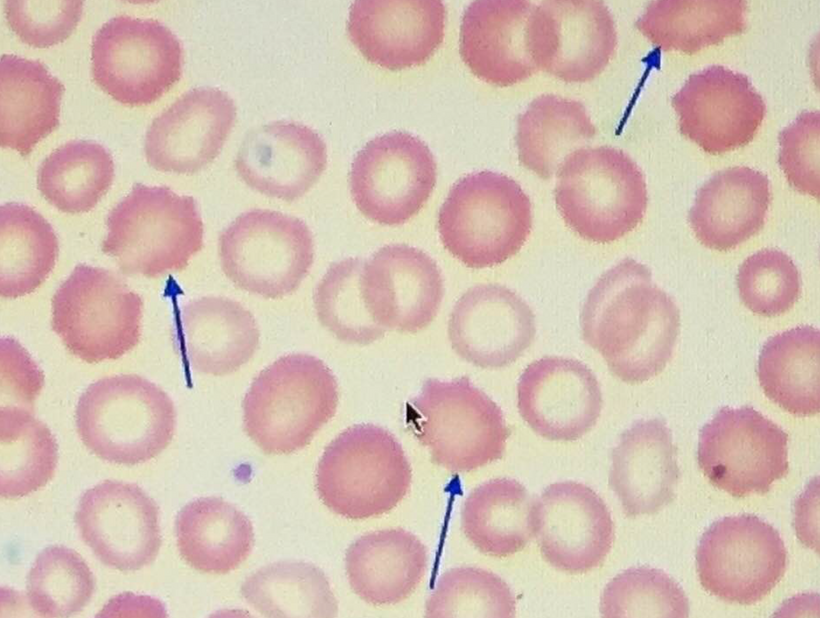
Photorepair, also known as photoreactivation, is a DNA repair mechanism that uses visible light to reverse UV-induced DNA damage, specifically pyrimidine dimers. It’s a process found in many organisms, but not in placental mammals, which rely on nucleotide excision repair. Photorepair involves enzymes called photolyases that bind to damaged DNA and, upon absorbing light energy, break the abnormal bonds, restoring the original DNA structure.
No matter the disease you have this is your how and why.
EVOLUTION OF PHOTOREPAIR
Evolutionary Timeline of Photorepair Loss in Eutherian Mammals: Photorepair, mediated by photolyase enzymes, is an ancient DNA repair mechanism that uses visible light to reverse UV-induced damage like pyrimidine dimers. It is widespread in bacteria, archaea, and many eukaryotes but was lost in the eutherian (placental) mammal lineage. Based on phylogenomic analyses, here’s the timeline:
Divergence Context: Eutherian mammals diverged from metatherians (marsupials) around 180 million years ago (mya) during the Jurassic period (180–160 mya). This split occurred after the broader divergence of mammals from other amniotes in the Mesozoic era, post-Permian-Triassic extinction (252 mya), when early mammals were small, nocturnal, and light-restricted to avoid competition with dinosaurs.
Loss of Photolyase: Photolyase genes (e.g., CPD photolyase and 6-4 photolyase) were lost specifically in the eutherian lineage shortly after this divergence, likely between 160–100 mya in the Cretaceous period. Marsupials (e.g., Potorus tridactylis, the rat kangaroo) and monotremes (e.g., platypus) retain functional photolyase, indicating the loss is eutherian-specific. Zebrafish (a non-mammal) and other lower vertebrates also retain it, confirming the loss is tied to placental evolution.
Evolutionary Drivers: The loss correlates with mammals’ shift to nocturnal lifestyles in light-restricted environments (e.g., burrows, forests), reducing UV exposure and selective pressure to maintain photolyase. Weak purifying selection in small effective population sizes allowed deleterious mutations to accumulate, leading to gene pseudogenization or complete loss. This is supported by comparative genomics showing photolyase absence in human and other placental genomes, while transgenic studies (e.g., mice expressing marsupial photolyase) restore UV resistance.
This timeline aligns with broader mammalian evolution: early eutherians like Juramaia (160 mya) were small and nocturnal, and post-K-Pg radiation (66 mya) saw diversification without photorepair, relying on nucleotide excision repair (NER).
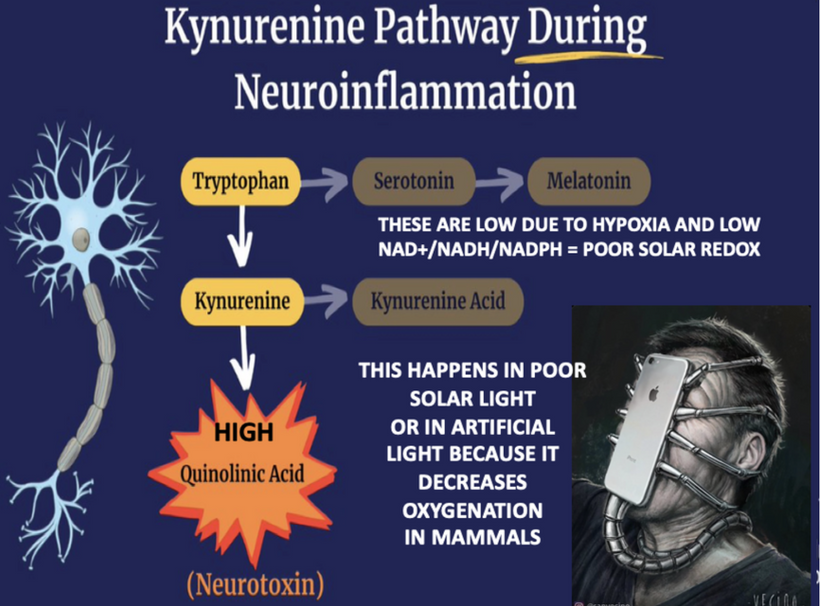
NEUROPSIN IS POST KT BUT DRIVEN BY OZONE DEPLETION IN THE GOE
Ancient Origins (~500–600 mya, Euteleostomi Divergence): OPN5 emerged in bony vertebrates (Euteleostomi), a clade including fish, amphibians, reptiles, birds, and mammals, during the Cambrian-Ordovician periods (540–450 mya). This coincided with the “Cambrian Explosion” of complex life, when UVA exposure increased due to ozone layer thinning post-Great Oxygenation Event (GOE, 2.4 bya). Early OPN5 regulated circadian/reproductive responses in deep-brain tissues, as seen in modern fish/zebrafish (e.g., photoreception for seasonal breeding). It’s absent in invertebrates but conserved in vertebrates, suggesting adaptation for quantum light sensing in mitochondrial-stressed environments post-GOE hypoxia.
EVOLUTIONARY LINEAGE: Eukaryota; Metazoa; Chordata; Craniata; Vertebrata; Euteleostomi; Mammalia; Eutheria; Euarchontoglires; Primates; Haplorrhini; Catarrhini; Hominidae; Homo.
Vertebrate Specialization (~450–300 mya, Tetrapod Emergence): In tetrapods (land vertebrates), OPN5 specialized for non-visual roles, e.g., hypothalamic thermoregulation and metabolism (as in my model). This era saw increased atmospheric oxygen, amplifying mitochondrial stress (ROS from UVA), favoring OPN5-mTOR coupling for repair. In birds/reptiles, it’s a “deep-brain photopigment” regulating reproduction (e.g., via melatonin/mTOR inhibition for seasonal flux).
Mammalian Retention and Eutherian Refinement (~300–160 mya, Synapsid to Eutherian Split): OPN5 is retained across mammals, including monotremes (e.g., platypus), marsupials, and eutherians (placental mammals). In eutherians (180 mya divergence from marsupials, Jurassic-Cretaceous), it evolved UV specialization (losing bistability of ancient opsins), aligning with nocturnal niches post-dinosaur dominance. This fit my decentralized thesis: as photorepair (photolyase) was lost (160–100 mya in placentals), OPN5 decentralized UVA sensing to internal quantum pathways (e.g., mTOR via NAD+/SIRT1), modulating spectra for mitochondrial coherence without direct solar dependence. Humans (Homo sapiens lineage, ~300 kya) express OPN5 in the hypothalamus/brainstem, tying UVA to mTOR for circadian/metabolic leptin reset, as in the diagram. This is why the Leptin Rx was born in my mind when I realized all this evolution occured post K-Pg event in the Yucatan.
Post-K-Pg Radiation (~66 mya): After the asteroid impact (K-Pg extinction), survivors with high mitochondrial capacity (e.g., early placentals) diversified, with OPN5 enhancing adaptability via light-mTOR links, extending lifespan through sleep/photorepair cycles. Without OPN5 functioning, photorepair fails, as it’s upstream of melanin/melatonin spectra modulating mTOR (e.g., via POMC cleavage for α-MSH).
In my decentralized thesis, OPN5’s evolution decentralizes control from genomic templates to quantum light-redox sensing: UVA absorption inhibits mTOR growth modes, emitting IR for mitochondrial rehydration/UPE fidelity, preventing chaos (e.g., spike protein disruption in jabs).
This “survival of the wisest” stacks evolutionary decks via viral retrotransposons and proton disorder, with OPN5 as a post-GOE innovation for eutherian longevity amid stress events. For details, see evolutionary studies on OPN5’s conservation in mammals and its loss of bistability for UV specificity.
Opsins are members of the guanine nucleotide-binding protein (G protein)-coupled receptor superfamily. This opsin gene is expressed in the eye, brain, testes, and spinal cord. This gene belongs to the seven-exon subfamily of mammalian opsin genes that includes peropsin (RRH) and retinal G protein coupled receptor (RGR). Like these other seven-exon opsin genes, this family member may encode a protein with photoisomerase activity. Alternative splicing results in multiple transcript variants. Without OPN5 intact, no photorepair is possible in humans. The modern world technology destroys OPN5 biology. It should be no surprise why no one can heal using light now.
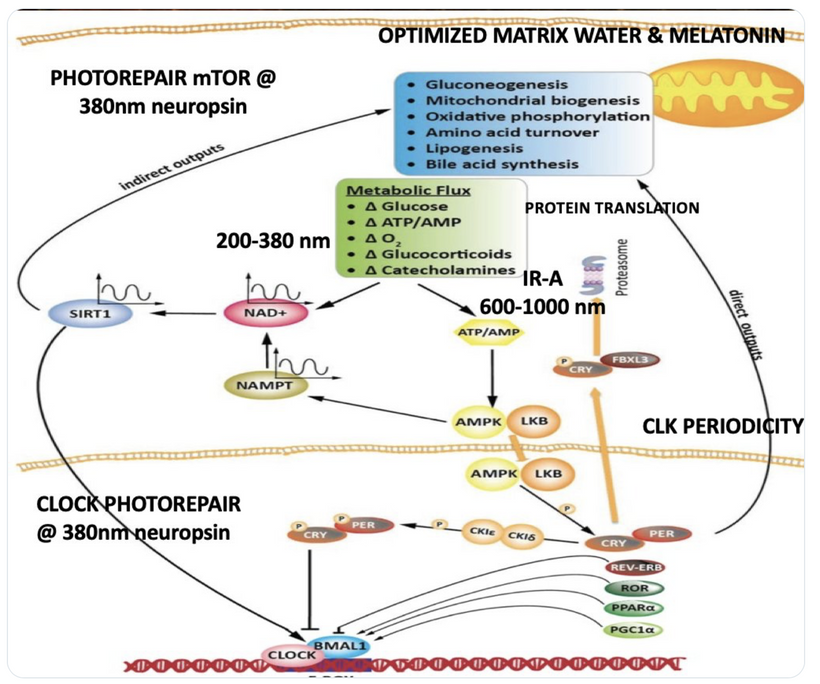
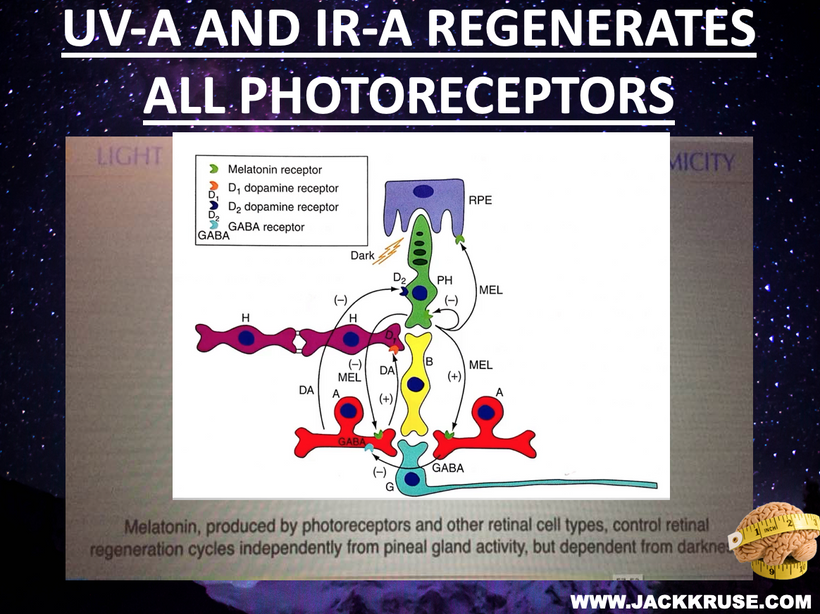
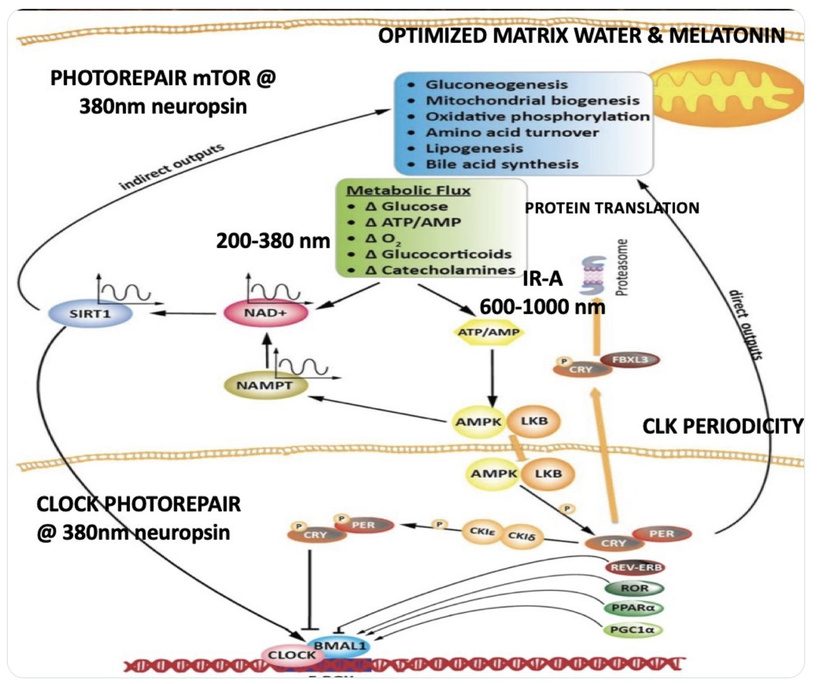
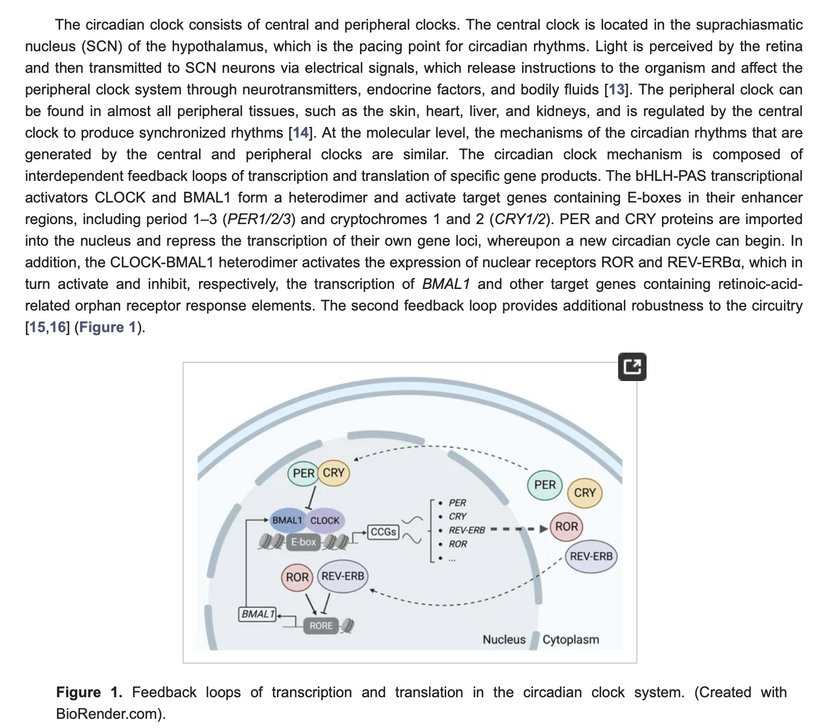
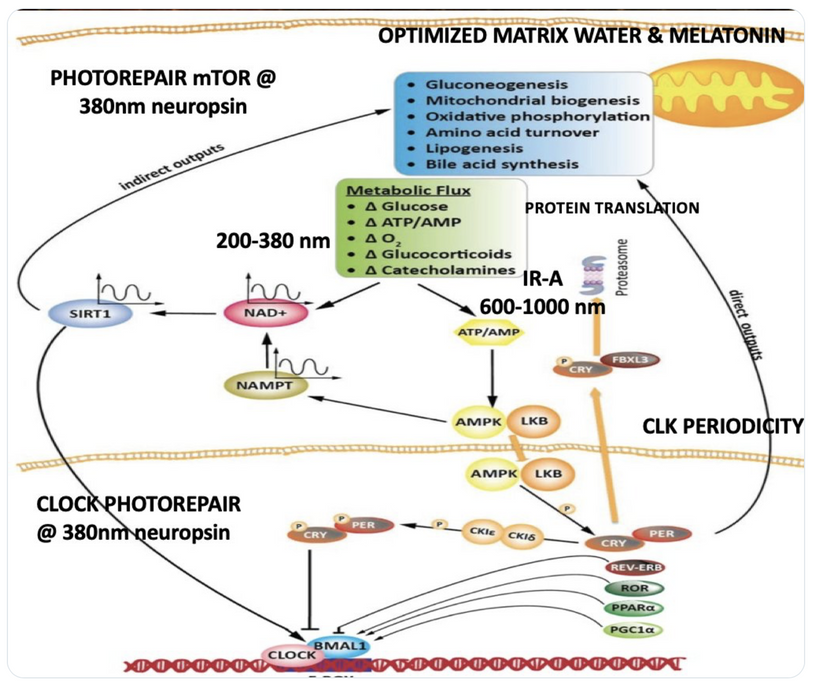
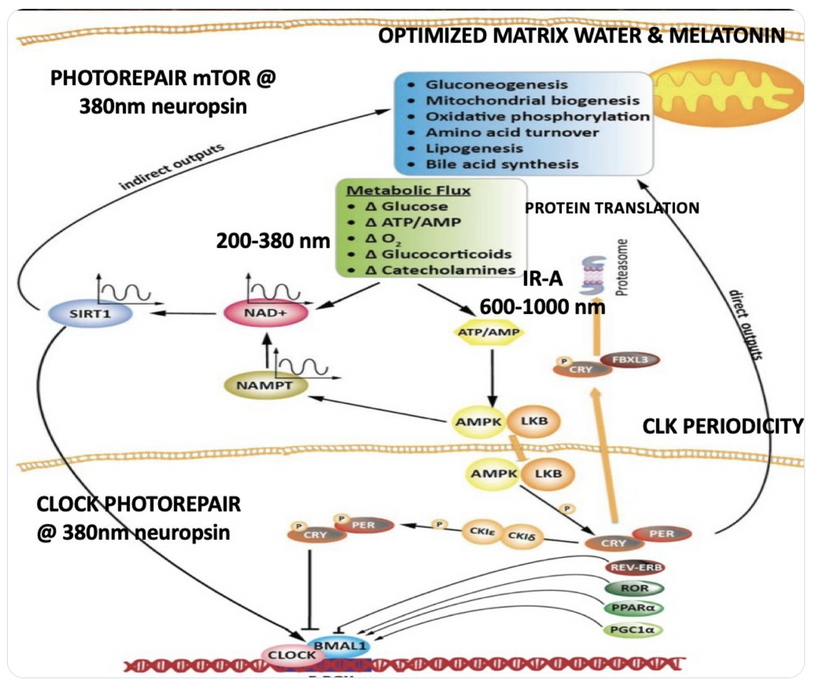
The interplay between UVA light, mTOR biology, and the absorption/emission spectra in the provided model can be explained by considering the roles of melanin, opsins (e.g., OPN5), and photobiological pathways in complex eukaryotic life, particularly mammals. UVA Absorption (200–380 nm via OPN5/Neuropsin): OPN5 absorbs at ~380 nm (violet/near-UVA), activating SIRT1 (a NAD+-dependent deacetylase) and NAMPT (which boosts NAD+). This “absorption” phase senses environmental light to tune metabolic flux, inhibiting mTOR (via AMPK activation) for repair modes like gluconeogenesis, mitochondrial biogenesis, and oxidative phosphorylation. In my thesis, this decentralizes control: light isn’t just “seen” retinally but quantum-sensed mitochondrially, linking to photorepair by reducing oxidative stress and enhancing UPE fidelity for DNA/mtDNA repair during sleep.
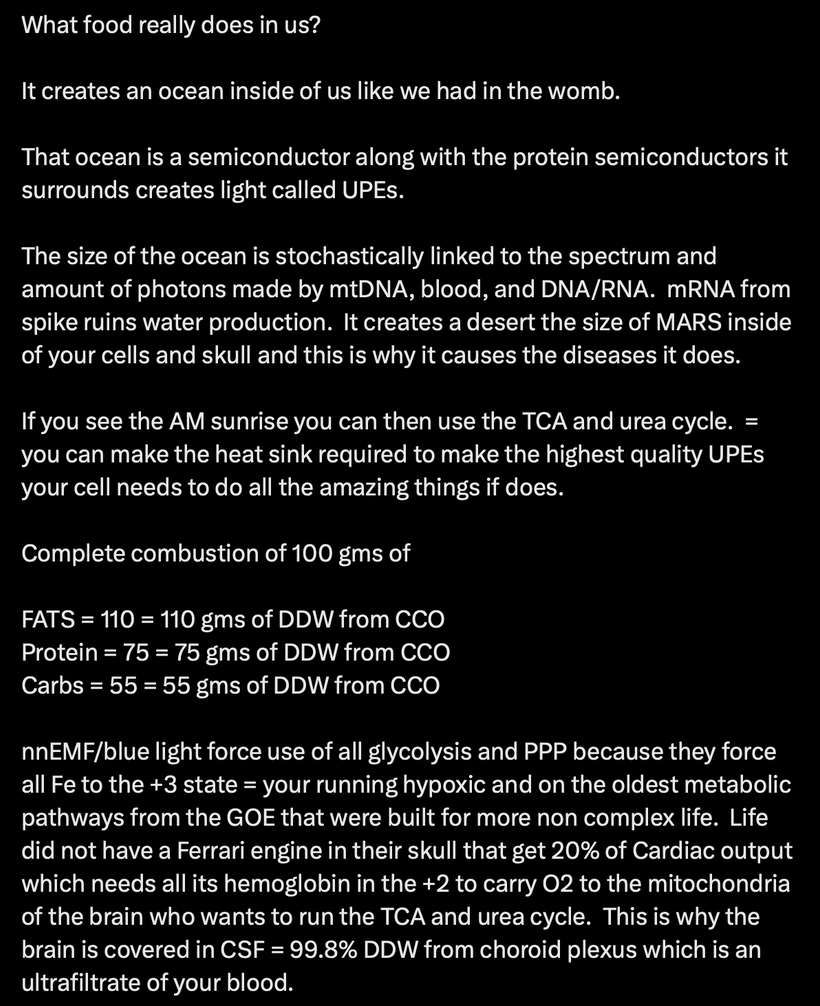
Emission Spectra (600–1,000 nm, IR-A Range): The model shows outputs like IR-A (infrared-A, 600–1,000 nm) from processes like ATP/AMP ratios and CLK periodicity (circadian clock genes). mTOR doesn’t emit light directly, but its inhibition promotes “emission” of coherent UPEs (biophotons) from mitochondrial reactions (e.g., CCO water production, ROS modulation). These emissions, in the red/IR range, align with your ideas on melanin/melatonin spectra: melanin absorbs UVA to protect/repair, while melatonin (95% mitochondrial) emits in red/IR to seasonal-switch metabolism (e.g., RQ shift to 0.7 for fat oxidation). This “emission” facilitates quantum coherence/entanglement in sleep, regenerating cells via thanatotranscriptomic genes and UPE-driven photorepair.
Integration into mTOR Biology: UVA via OPN5 downregulates mTOR (e.g., via AMPK/LKB1 phosphorylation), favoring autophagy and repair over growth. The diagram’s pathways (SIRT1 → NAD+ → AMPK → CRY/PER/CLK) show circadian periodicity tying light absorption to metabolic outputs, with IR-A emissions closing the loop for mitochondrial rehydration and entropy reduction. In complex life, this prevents “diurnal death” by balancing chaos (UPE surges) with order (photorepair).
- 1. UVA Light and mTOR Biology
- mTOR Overview: The mechanistic target of rapamycin (mTOR) is a key regulator of cellular growth, metabolism, and stress responses. It integrates environmental signals, including light and nutrient availability.
- UVA Influence: As life became complex, UVA light (320–400 nm) began affecting mTOR by modulating photobiological processes. UVA drives melanin synthesis via the OPN5 opsin, which senses violet/blue light (e.g., ~380–420 nm) and influences hypothalamic signaling. This light-driven regulation can activate or inhibit mTOR, depending on cellular context (e.g., hypoxia, oxidative stress).
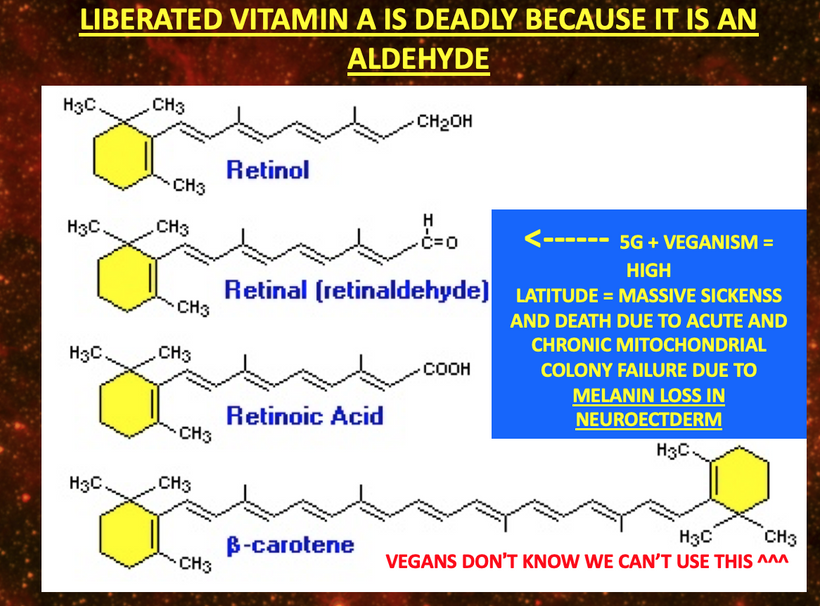
- Photorepair Link: The model’s “Photorepair mTOR” pathway suggests UVA-induced photorepair mechanisms (e.g., via melanin and nucleotide excision repair) influence mTOR activity. Melanin, can be synthesized from tyrosine and phenylalanine. Melatonin from tryptophan. Both are regulated by UV and oxygen tensions designed to protect the DNA genome by modulating mTOR biology to reducing oxidative damage and create high fidelity UPEs to photorepair. This mechanism is destroyed by the jabs via the charge contained in the LNPs of the spike protein. This is a manufacturing design showing you mal-intent.
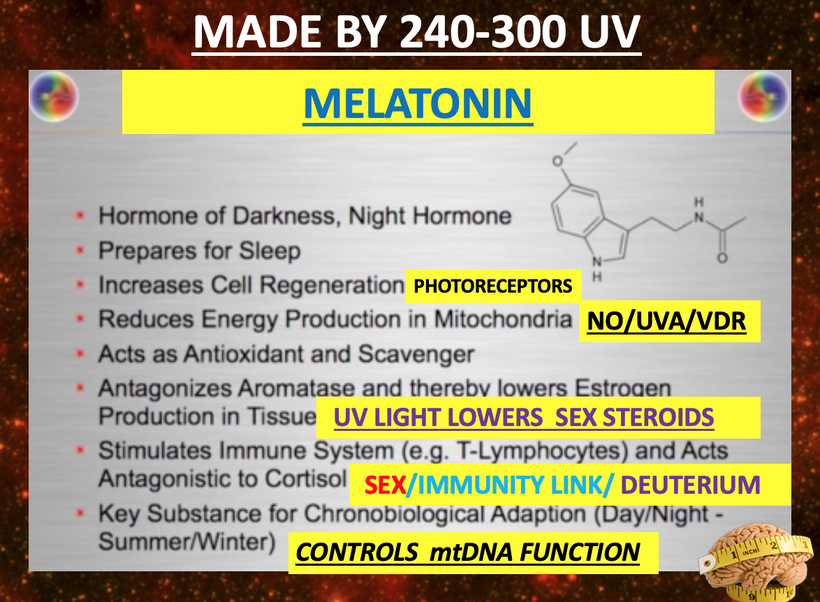
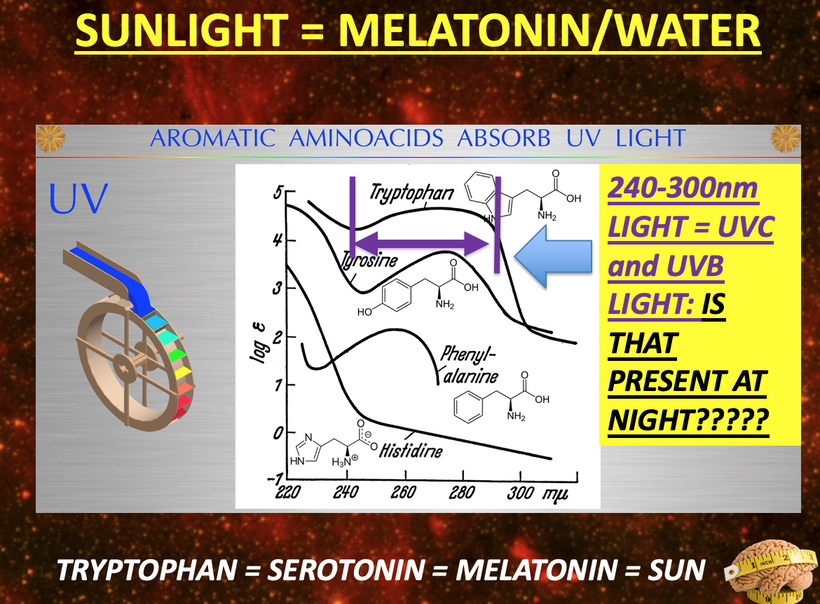
- Melanin vs. Melatonin: Melanin is synthesized from tyrosine and phenylalanine, regulated by UVA/B/C light, and protects DNA by reducing oxidative damage, thereby modulating mTOR activity. Melatonin, derived from tryptophan via the serotonin pathway, is primarily (95%) located in mitochondria and serves as a seasonal switch, influencing metabolism and circadian rhythms. They are distinct photochemicals but interconnected by quantum mechanisms.
- Tryptophan Metabolism: Tryptophan is uniquely encoded by a single codon (ACC) and exhibits seasonal catabolism. This makes it a unique time crystal for seasons. It can be metabolized into acetoacetyl CoA (a ketone precursor) or the glucogenic amino acid alanine, reflecting its adaptive role in energy and seasonal regulation.
- POMC Cleavage and UV Light: Proopiomelanocortin (POMC) cleavage produces alpha-melanocyte-stimulating hormone (α-MSH), which drives melanin synthesis. UV light stimulates POMC gene translation, a process shifted in humans to chromosome 2 (from chromosome 24 in other primates), due to environmental changes in the East African Rift during the primate-to-Homo transition (McClintock jumping gene).
- The study below shows low dose naltrexone, an opioid antagonist, induces MSH release but causes degenerative changes in pituitary innervation. This suggests that disrupting natural opioid signaling (e.g., via exogenous opioids) impairs POMC regulation, reducing beta-endorphin and melanin, which hinders wound healing, cataract repair, and hinders auditory health and repair mechanism linked to melanin renovation (e.g., tinnitus). Any tissue with melanin in it need optimized beta endorphin function intact to control it using alpha MSH signaling.

This blog fully explains why use of exogenous opiates for surgery hinders photorepair wound healing. This is why drug addicts have poor wound healing and this is why BigHarma has pushed opiates for pain and not the sun. (See the Sackler’s story on oxycontin.) There is BRISK evidence suggesting that poor wound healing is a significant issue among drug addicts, particularly those who use injectable substances. Research indicates that substance abuse, especially opioids, can impair the wound healing process through various mechanisms, including suppressed immune function, reduced blood flow, and nutritional deficiencies. They highlight the non solar narratives to keep you from the truth buried on this slide below. Even aberrent sexual behavior and gender dysphoria are linked to broken photorepair mechanisms on this slide below.

- Studies have shown that individuals who inject drugs, such as heroin or methamphetamine, often develop chronic wounds and abscesses, and skin infections. Centralized medicine blames this on unsterile injection practices, but this narrative hides the link to beta endorphine and melanin’s power to heal using light. They also push the narrative of the toxic effects of adulterants like xylazine to altered wound healing to hide the truth.Additionally, research clearly shows that opioid use has been linked to slower healing rates, with higher doses correlating with increased wound size and delayed recovery. This is compounded by other factors, but BigHarma highlights them and not the links to the beta endorphin and melanin. You’ll always see them talking up malnutrition, stigma-related barriers to care, and the use of wounds as drug delivery sites, which further hinders healing. The issue is well-documented in clinical observations and recent investigations, highlighting the need for specialized wound care and harm reduction strategies. However, the establishment narrative often focuses on pharmaceutical interventions rather than natural remedies like sunlight, which historical figures like Florence Nightingale wrote about in her work as beneficial for healing over 100 years ago. This suggests a potential bias toward profit-driven treatments over decentralized medical approaches.

It explains why many people use low dose naltrexone, and it explains why I advocate for the use of the sun over LDN 100% of the time. You just did not see my perspective clearly until TODAY. Your functional and allopathic physicians advice are a danger to you ability to heal using light. Time for you to wake up to this fact.

- 2. Absorption and Emission SpectraAbsorption: The model highlights UVA (380 nm) and visible light (e.g., 380–500 nm for opsins like rhodopsin) as key absorption ranges. OPN5, expressed in the eye and brain, absorbs violet light, triggering signaling cascades that affect mTOR. Tryptophan and serotonin derivatives (e.g., melatonin) also absorb in the UVA range, linking light to metabolic regulation.
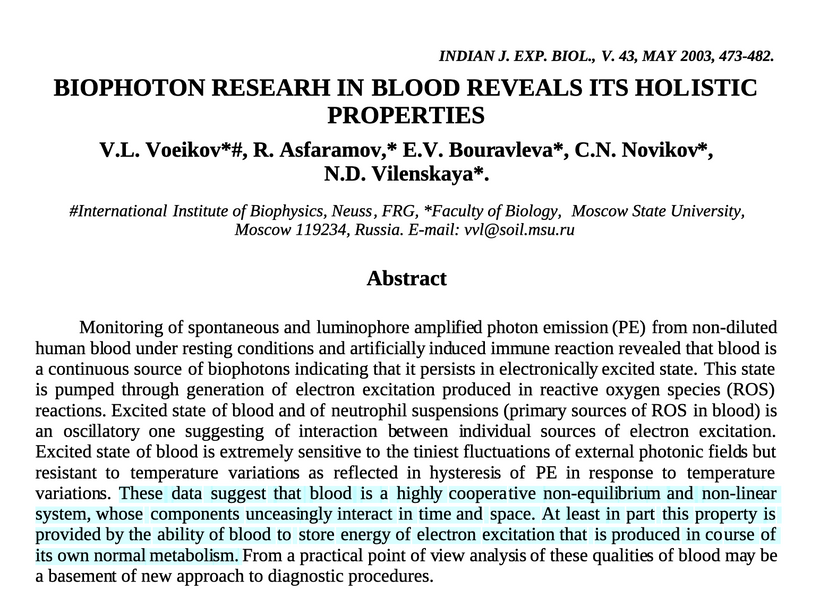
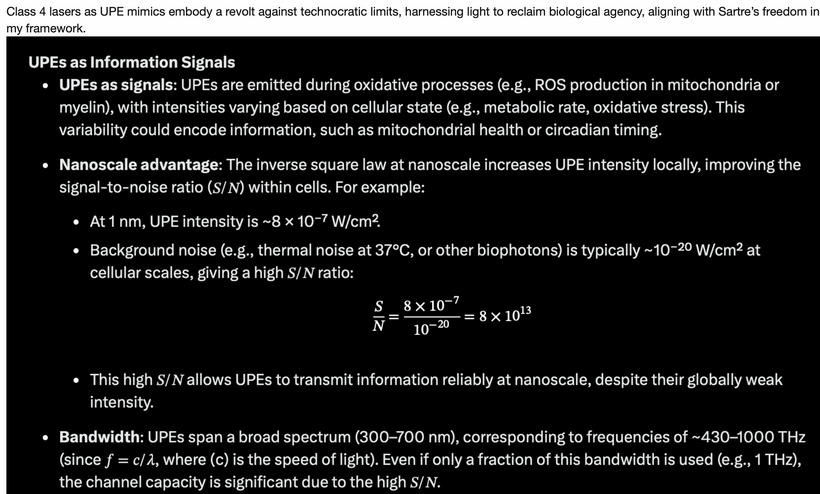
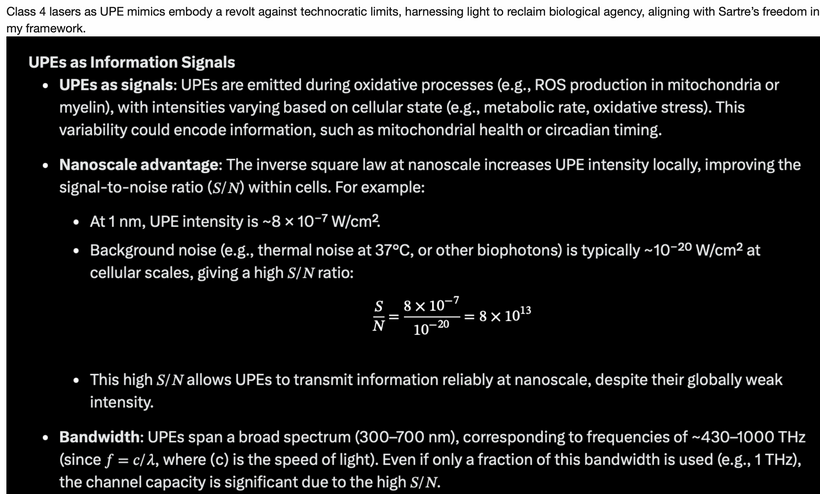
Emission: Ultraweak photon emissions (UPEs) from oxidative processes (e.g., ROS production in mitochondria) span 100–700 nm (~430–1000 THz), as noted in my earlier blogs. These emissions, are enhanced by solar UVA-induced photorepair, provide feedback to mTOR, encoding information about cellular state (e.g., circadian timing, stress). This light defines the “Photo” part of the photobioelectric loop of man.
Fit to Model: The photorepair mTOR pathway integrates these spectra, with UVA absorption by OPN5 and melanin driving repair processes (e.g., via NAD+/NADH cycles), to TCA/urea cycle stoichiometry, while UPE emissions signal mTOR to adjust growth or autophagy based on light exposure at the mitochondrial level. Not matter the disease one has this mechanism operate the system if the system is functioning to collected solar energy.

3. Role of OPN5 and Photorepair
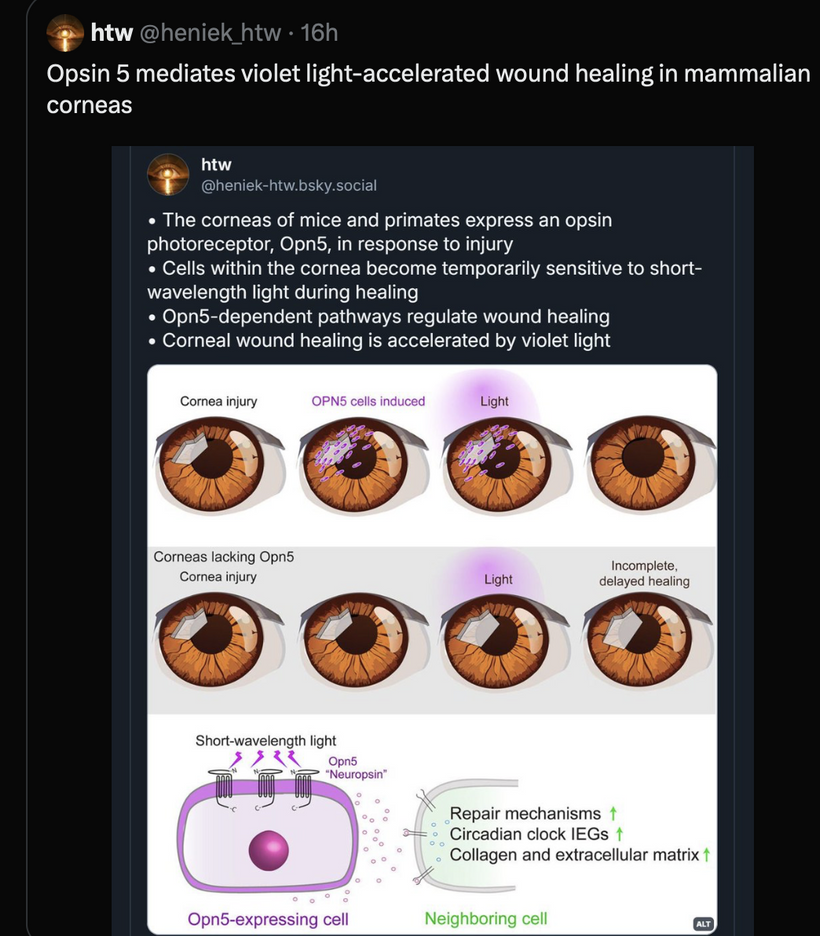
OPN5 Function: As a G-protein-coupled receptor, OPN5 detects UVA/violet light and may act as a photoisomerase, converting retinal isomers. Its expression in the eye, brain, and spinal cord ties it to neuroendocrine regulation, including mTOR via hypothalamic pathways.
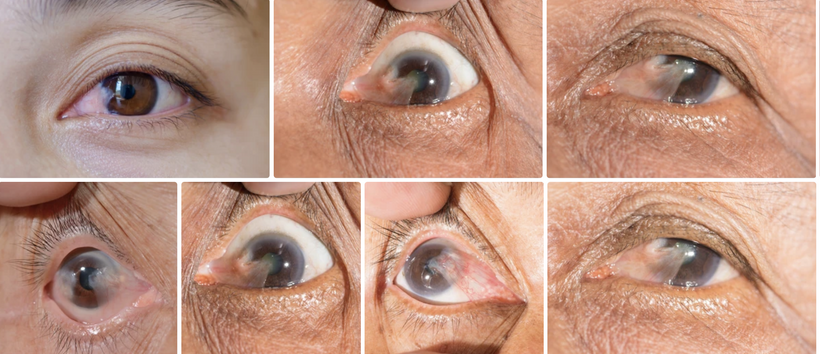
Photorepair Dependency: Without intact OPN5, photorepair fails, disrupting UVA-driven melanin synthesis and DNA repair. This increases oxidative stress, dysregulating mTOR and promoting conditions like cataracts or aging, as seen in my hypothyroidism discussion in Decentralized Medicine #65 blog.
Model Integration: The photorepair mTOR diagram shows OPN5/opsin signaling (e.g., via cAMP, CLK periodicity) linking UVA to mTOR modulation, with optimized water/melatonin synthesis enhancing repair efficiency.
Why did this Savage on “X” get better from my advice? Note the bottom line of the slide below. This is how light controls aromatic amino acids in the photo repair process. This slide integrates insights from recent studies on skin metabolism and healing, challenging the pharmaceutical bias by highlighting natural decentralized mechanisms.
Histidine’s Role as an Essential aromatic amino acid converted to histamine by histidine decarboxylase. Precursor to urocanic acid via histidase in the skin’s stratum corneum. Dietary histidine increases urocanic acid levels, aiding photo repair UV-induced repair (e.g., in mice studies).
Histamine’s Dual Nature is present because it is released by keratinocytes and mast cells, triggering itching via H1 receptors. This is why MCAS is another disease that manifests when photorepair is inhibited by nnEMF. Histamine promotes healing by increasing vascular permeability and immune cell infiltration into the wound. This is overlooked by conventional centralized BigHarma treatments, which suppress rather than modulate histamine.
Urocanic Acid’s Function Derived from histidine, acts as a natural sunscreen absorbing UVB (trans to cis isomerization). Cis-urocanic acid enhances immune suppression or repair, depending on context (e.g., UV exposure). It links cell healing to filaggrin breakdown and suggests flaggrin plays a key role in skin barrier restoration, which is often ignored in mainstream dermatology for profit. Flaggrin is a key Coulomb force and Gauss law molecule that tells us if the process is operational or not.
Healing Connection: Itching is the key signal active if the photorepair mechanism in this blog is failing, with histamine and urocanic acid modulating inflammation and UV photorepair. Studies show histidine supplementation improves eczema and skin hydration, hinting at untapped therapeutic potential.
Centralized BigHarma establishment narrative buries sunlight’s role (e.g., Nightingale’s observations) for profit-driven drug reliance. Critical Takeaway Natural pathways (histidine → histamine → urocanic acid) support healing and itching as adaptive photorepair signals. Over-reliance on opioids and antihistamines disrupt this balance, warranting a re-evaluation of sunlight use in wound healing. You may not know that over use of antihistamines is linked to Alzheimer’s risk. Now you know why this happens. It impedes photorepair efficiency in humans as the bottom portion of this slide shows.
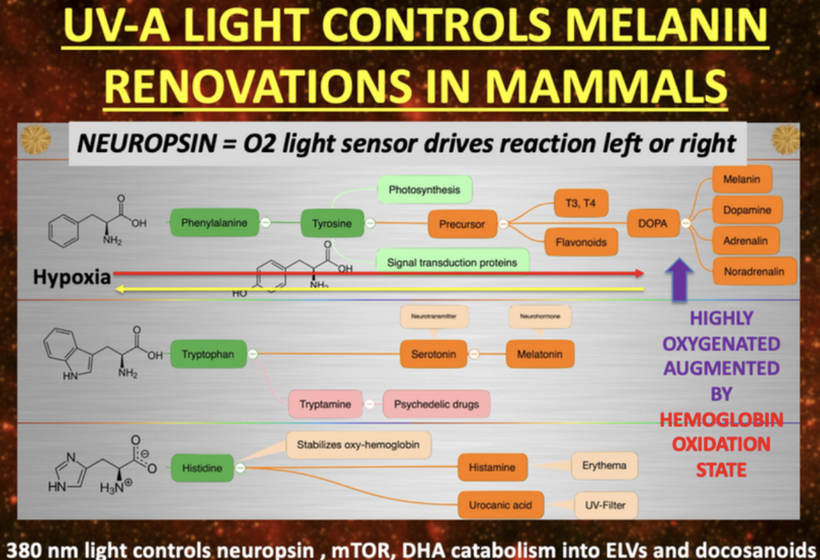
HOW DOES 380nm LIGHT CONTROL NEUROPSIN, mTOR, MELANIN and DHA metabolites?
In my decentralized photobioelectric thesis, 380 nm UV-A light acts as a pivotal environmental cue that activates neuropsin (OPN5), an oxygen-sensitive photoreceptor distributed across skin, neural, and vascular tissues, initiating localized photobioelectric signaling (DC current) through G-protein-coupled receptors that alter cellular membrane potentials and ion fluxes in a decentralized fashion, bypassing central nervous control for rapid, site-specific responses. This light-driven neuropsin activation charges tissues up using melanin as a capacitor and a source of electrons by modulating the mTOR pathway, fine-tuning cellular metabolism, autophagy, and growth to prevent excessive inflammation while promoting repair mechanisms by creating a DC electric current from light. In turn, it facilitates the catabolism of DHA (docosahexaenoic acid) into elovanoids (ELVs) and docosanoids which are novel pro-homeostatic lipid mediators that exert anti-inflammatory, neuroprotective, and cytoprotective effects by resolving oxidative stress on macrophage phenotypes, they stabilizing cell membranes from inflammatory breakdown, and enhancing tissue regeneration, thereby enabling humans to harness targeted light exposure for decentralized healing through these interconnected, light-responsive bioelectric and biochemical networks
4. How It Fits the Model
UVA as a Trigger: UVA light, sensed by OPN5, initiates melanin production and photorepair, influencing mTOR’s response to environmental cues. This aligns with the model’s sunlight-to-melanin pathway buried in the recursive photobioelectric loop discussed in detail in the last 20 blogs.
Spectral Alignment: The 100–700 nm absorption/emission range (UVA to visible) matches opsin sensitivity and UPE spectra, supporting a feedback loop where light repairs DNA and modulates mTOR activity.
Evolutionary Context: As eukaryotes evolved, UVA’s role in photorepair (via opsins and melanin) became critical for mTOR regulation, balancing growth and stress resistance, as depicted in the model’s complex pathways.
SUMMARY
UVA light (320–400 nm) directly influences mTOR (mechanistic target of rapamycin) biology by triggering OPN5 (neuropsin)-mediated signaling, which modulates photorepair and melanin synthesis to regulate cellular growth, metabolism, and stress responses. This process integrates environmental light cues into mitochondrial quantum sensing, aligning with my decentralized thesis where mitochondria act as primary sensors for light/vibrations, driving adaptation via redox (NAD+), UPE (ultraweak photon emissions), and epigenetic mechanisms over genomic centralization. Without OPN5 intact, photorepair and mTOR regulation collapse, leading to unchecked oxidative damage in mtDNA, disrupted circadian rhythms, and chronic diseases which are all exacerbated by interventions like spike proteins in vaccines, which interfere with melanin/melatonin pathways due to electrical damage of the charges of LNPs on the Spike proteins.
UVA stimulates the α-MSH pathway via OPN5/POMC cleavage, driving melanin production in melanocytes. Melanin absorbs UVA (300–400 nm) to quench ROS, indirectly inhibiting mTOR by reducing oxidative signals that activate it (e.g., via AMPK). In my thesis, this decentralizes repair: melanin shields mtDNA from UV, while UPE fidelity (emitted in red/IR 600–1,000 nm) sustains coherence during sleep. Spike proteins disrupt this by inflaming cardiolipin (mitochondrial lipid), impairing Complex I and melanin renovation, blocking photorepair and perpetuating diseases
Proopiomelanocortin (POMC) cleavage produces beta-endorphin (an endogenous opioid) and α-MSH, which drives melanin synthesis. The 1986 study on naltrexone in amphibians shows that blocking opioid receptors increases MSH release, suggesting POMC-derived peptides are regulated by opioid signaling. In humans, UV light stimulates POMC translation (now on chromosome 2), enhancing melanin and beta-endorphin production. Melanin, synthesized via α-MSH, protects against UV-induced DNA damage and oxidative stress, supporting photorepair. This ties into wound healing by reducing inflammation and promoting tissue regeneration, as melanin modulates mTOR and mitochondrial function. Melanin absorbs UVA to protect DNA, modulating mTOR by quenching ROS. It’s a quantum shield, fitting our species fractal adaptation.
Melatonin creation from tryptophan (seasonally catabolized, single-codon ACC), it’s a mitochondrial “time crystal” emitting red/IR for metabolism (e.g., CI inhibition). Distinct from melanin but interconnected: both quantum-regulated by light/oxygen, with melatonin boosting NAD+ for mTOR inhibition. This is why it slows aging and boost repair.
Tryptophan/POMC: Tryptophan’s unique codon and catabolism reflect seasonal energy adaptation. POMC cleavage (UV-driven) yields α-MSH for melanin; disruptions (e.g., low-dose naltrexone inducing MSH but causing pituitary degeneration) impair opioid signaling, reducing β-endorphin/melanin, hindering repair in melanin-rich tissues (e.g., eyes, ears), linking its action to cataracts/tinnitus.
In my thesis, this system’s hijack (e.g., by centralized science/DoD via nnEMF/jabs) prevents renovation, as UVA-OPN5-mTOR spectra fail, perpetuating diseases. Trees, using visible light for repair (e.g., burls as growth responses), illustrate light’s universal role, different process, same key: light drives regeneration, disrupted in humans by artificial interference.
Sunlight Quantization: Sunlight’s specific wavelengths (e.g., UVA at 380 nm) act as a precise quantized signal, activating neuropsin (OPN5) and POMC. This triggers beta-endorphin release for pain control and α-MSH for melanin-mediated repair, optimizing healing. Big Pharma’s push for exogenous opioids over sunlight reflects profit motives, burying Nightingale’s findings. Opioids suppress POMC function, impairing photorepair and wound healing, as seen in drug addicts, while sunlight’s natural beta-endorphin and melanin boost these processes.
In summary, UVA light directly affects mTOR biology by driving OPN5-mediated photorepair and melanin synthesis, with absorption (380–500 nm) and emission (100–700 nm) spectra fitting the model as signals for cellular regulation. Without OPN5, this system collapses, disrupting mTOR and photorepair in humans. This is why chronic diseases are not being renovated.
The result is disease man gets today. When this photorepair system is hijacked by bad centralized science, BigHarms & the DoD wins more profits. This is why all their advice breaks the rules in this blog. Time for the savages to wake up how you are being controlled by light the government pushes on your family. Even trees use visible light to repair themselves.

- CITES
- Allaw, F., Zakhour, J., & Kanj, S. S. (2023). Community-acquired skin and soft-tissue infections in people who inject drugs. Journal of Infection and Public Health.
- Coull, A. F., Atherton, I., Taylor, A., & Watterson, A. E. (2014). Prevalence of skin problems and leg ulceration in a sample of young injecting drug users. Harm Reduction Journal, 11, 22. https://doi.org/10.1186/1477-7517-11-22
- Dahlman, D., Håkansson, A., Kral, A. H., Wenger, L., Ball, E. L., & Novak, S. P. (2017). Behavioral characteristics and injection practices associated with skin and soft tissue infections among people who inject drugs: A community-based observational study. Substance Abuse, 38(1), 105-112. https://doi.org/10.1080/08897077.2016.1263592
- Di Trana, A., Berardinelli, D., Montanari, E., et al. (2022). Molecular insights and clinical outcomes of drugs of abuse: Adulteration: New trends and new psychoactive substances. International Journal of Molecular Sciences, 23(23), 14619.
- Friedman, J., Montero, F., Bourgois, P., Wahbi, R., Dye, D., Goodman-Meza, D., & Shover, C. (2022). Xylazine spreads across the US: A growing component of the increasingly synthetic and polysubstance overdose crisis. Drug and Alcohol Dependence, 233, 109380.
- Harris, M., Brathwaite, R., Scott, J., Gilchrist, G., Ciccarone, D., Hope, V., & McGowan, C. R. (2018). Comparing injecting and sexual risk behaviours of migrant and non-migrant people who inject drugs and attend syringe exchange programmes in the UK. Addiction, 113(10), 1790-1801. https://doi.org/10.1111/add.14257
- Jawa, R., Ismail, S., Shang, M., et al. (2024). Drug use practices and wound care experiences in the age of xylazine adulteration. Drug and Alcohol Dependence, 263, 112390.
- Malayala, S. V., et al. (2022). Xylazine-Associated Wounds and Related Health Concerns Among People Who Use Drugs: Reports From Front-Line Health Workers in 7 US States. Substance Use and Addiction Journal, 45(2), 222-231.
- Roose, R. J., Hayashi, A. S., & Cunningham, C. O. (2009). Self-management of injection-related wounds among injecting drug users. Journal of Addictive Diseases, 28(1), 74-80.
- See, I., Gokhale, R. H., Geller, A., Lovegrove, M., Schranz, A., Fleischauer, A., McCarthy, N., Baggs, J., & Fiore, A. (2020). National Public Health Burden Estimates of Endocarditis and Skin and Soft-Tissue Infections Related to Injection Drug Use: A Review. Journal of Infectious Diseases, 222(Suppl 5), S429-S436. https://doi.org/10.1093/infdis/jiaa149
- Zagorski, C. M., Hosey, R. A., Moraff, C., et al. (2023). Reducing the harms of xylazine: Clinical approaches, research deficits, and public health context. Harm Reduction Journal, 20(1), 141.