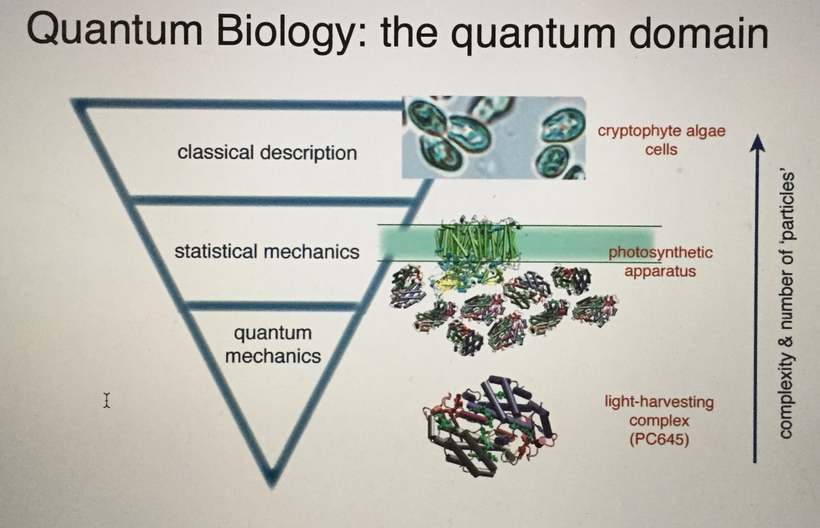
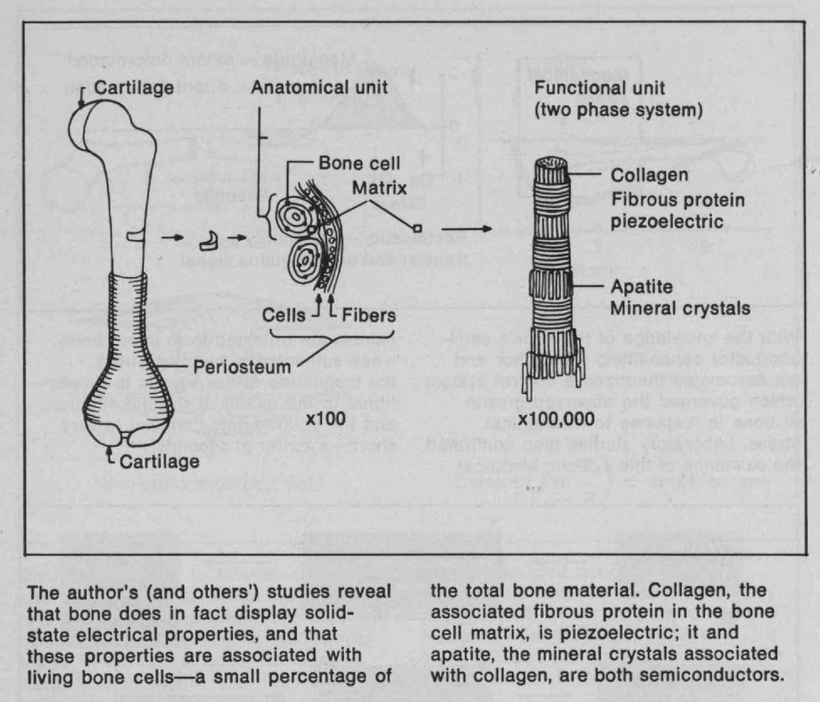
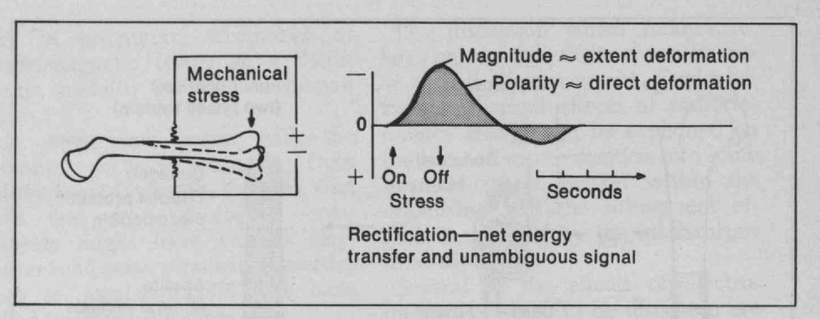
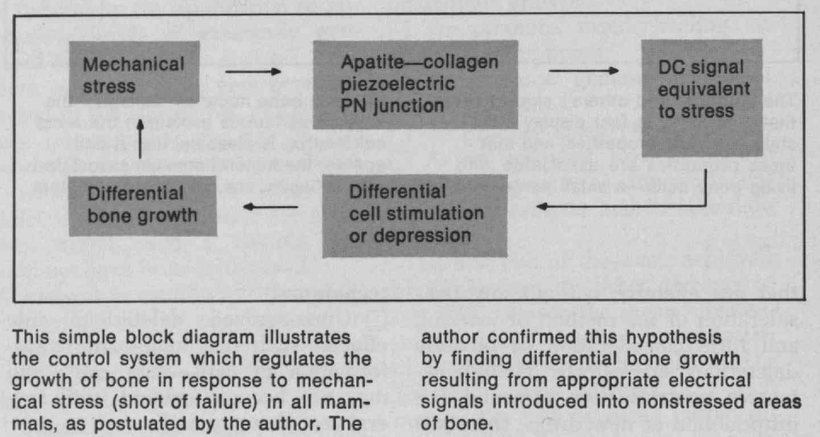
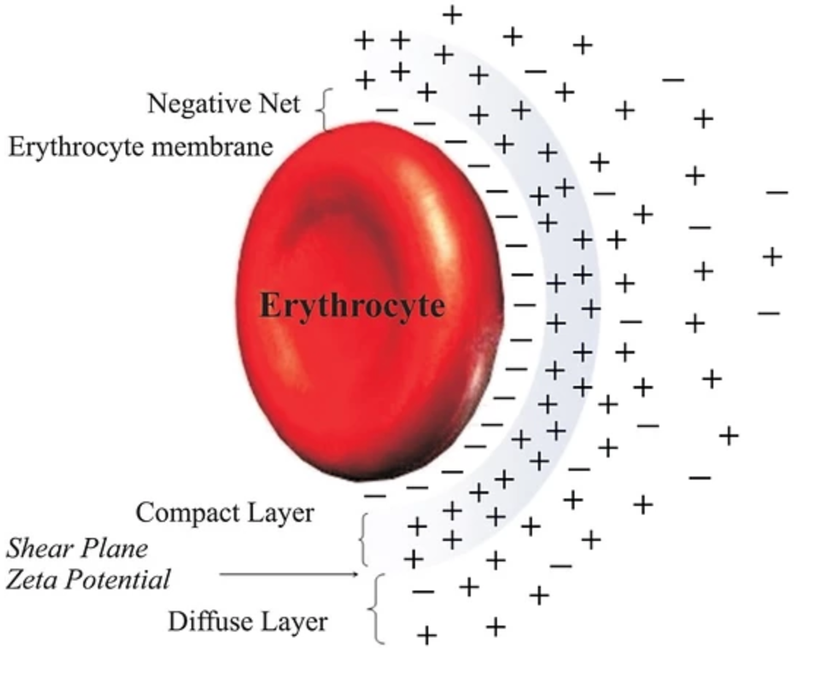
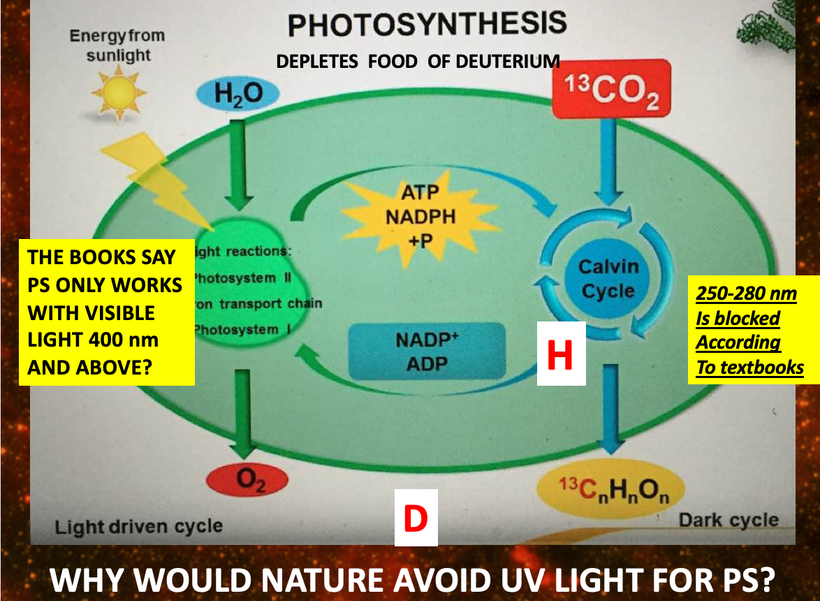
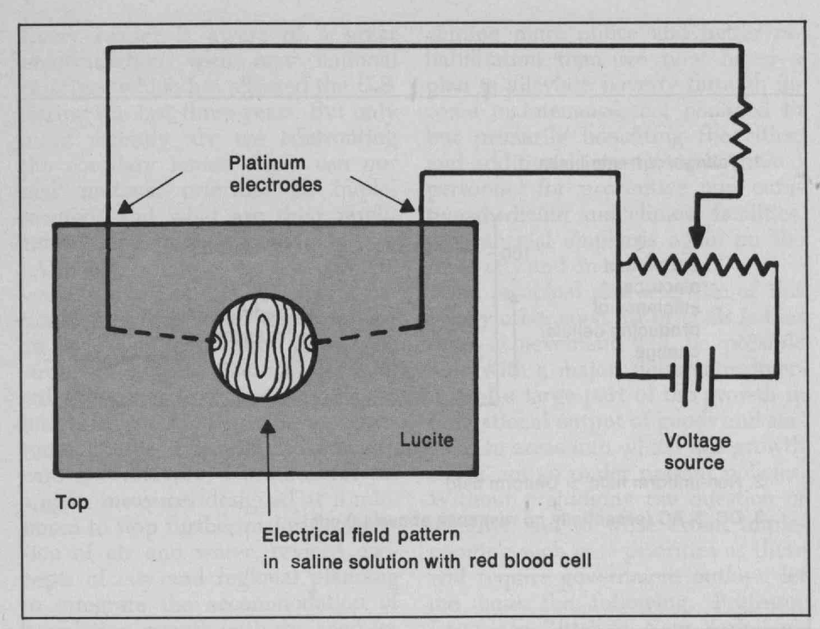
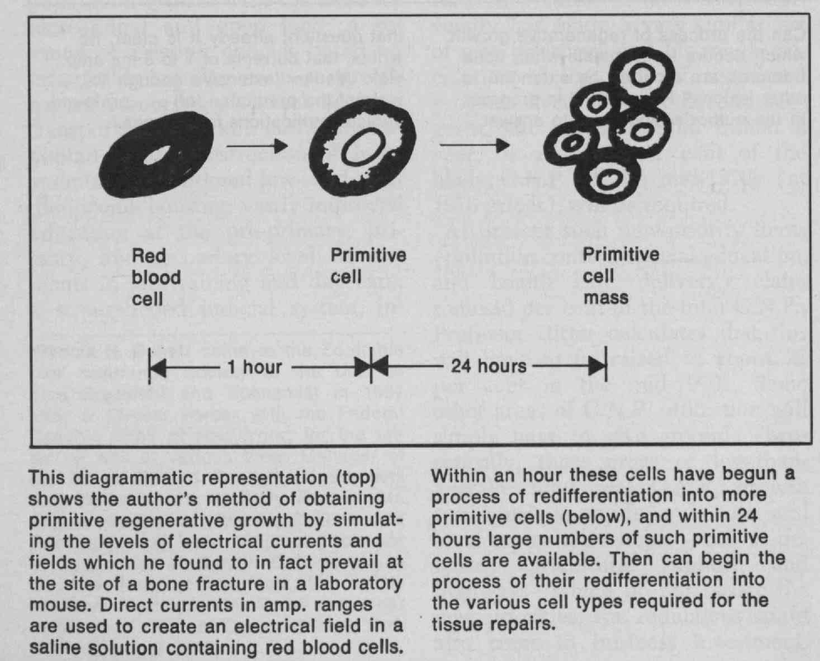
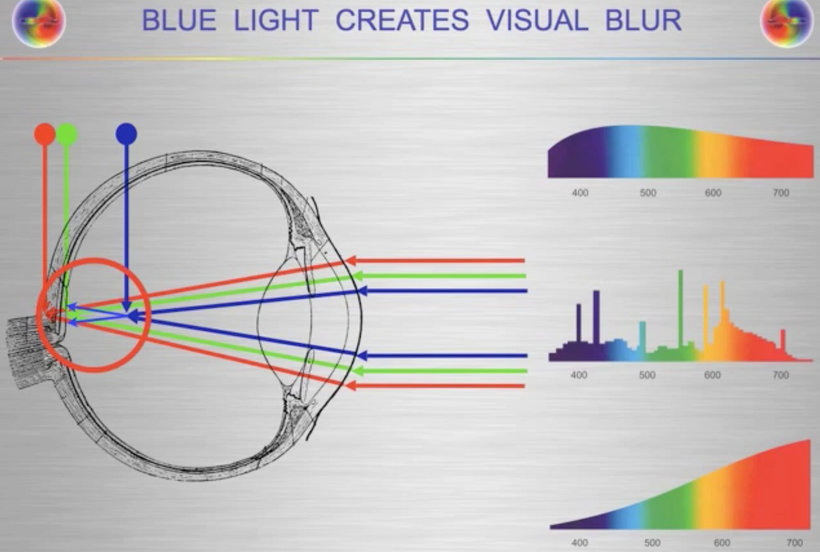
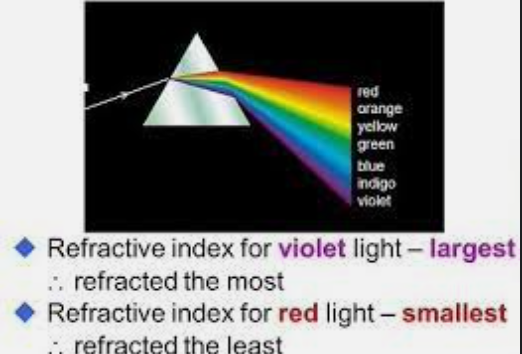
Do you know humans are broken and moved to action by their master?
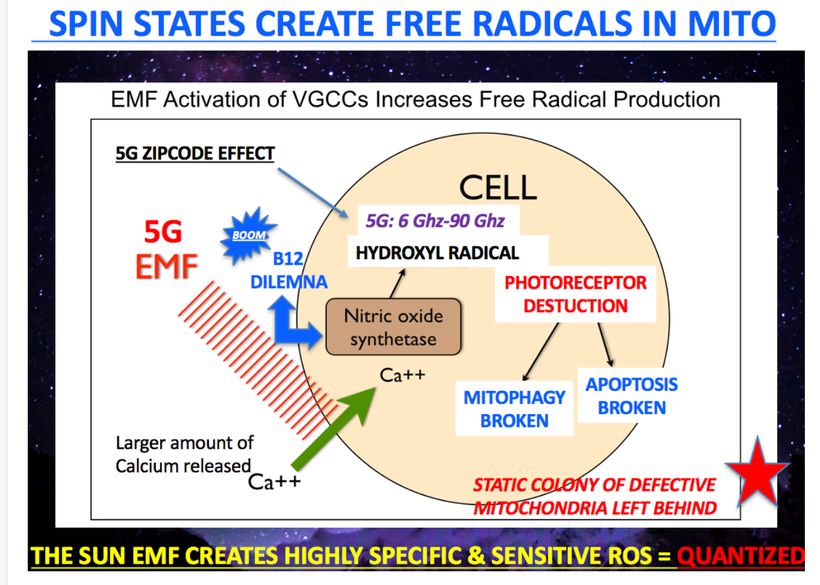
What are minerals at their most fundamental level? Atoms. What are they made of? Electrons, protons, and neutrons have their quantum spin, and the angular orbital momentum is changed by light from the sun. Is this transferred to water and things in our cells? Yep. This is how evolution really operates below your level or a Darwinist to sense it
.
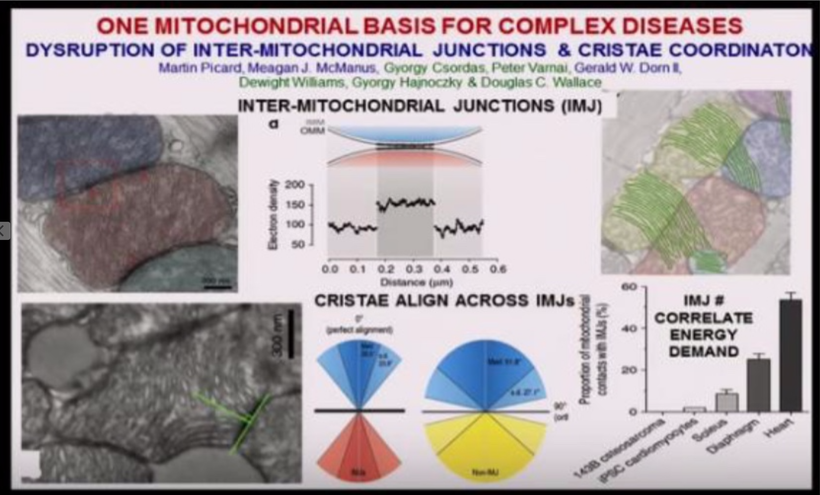
Why does just 3% of DNA code for proteins? How could it be the be-all-end-all that the neo-Darwinists say it is? What does the other 97% of DNA do? Is the extra DNA that acts like dark matter in us actually an owner’s manual for how light, our master, is transformed into bioelectricity whose job it is to power up and move the chains found in our proteins to move like a wind chime operates to make music? Is bio-electric function vital to understanding how our master controls the dark matter in us to allow life to manifest somehow, and is centralized science missing?

DNA only codes for proteins. Protein structure is the three-dimensional arrangement of atoms in a molecule. Proteins are polymers of specifically polypeptides formed from sequences of amino acids that the linear DNA code provides. The code of DNA, however, has many other electronic codes contained within it. Watson and Crick did not understand this in 1953, and many in centralized biology are still stuck on their old ideas. Modern decentralized epigenetics and transposons are tuned and turned on and off by bioelectrical signals transformed from sunlight, which have shown us that Watson and Crick’s ideas about DNA are outdated. This implies that the DNA code and proteins it makes are clues to a “quantum evolution” at the depths of life deep inside your cells.

The design process of life begins with the DNA code. Biology today is a detailed, disorganized collection of disparate facts. It is like a hoarder’s basement or a rat’s nest. There is no design connecting to what is buried in DNA’s code. You can scoop up a bag full of facts and try to make sense of it, but that would be an exercise in futility. Proper biological knowledge is fractal and non-linear, filled with optical magics thrown in the recipe.

The design can be complex, with microscopic details, but the overall design is coherent and beautiful. It would help if you linked them together electrically to make extensive collections of proteins act as one and become coherent. This idea implies that they must be tuned as a wind chime is. Cells should have some bioelectric tuning ability built into their protein structure. Do you see what I see yet?
VIDEO
Just one violin that is out of tune in an orchestra or one voice that is off-key in a choir will ruin a beautiful harmony or an enchanting ensemble. It matters not how loudly or softly it grates; if it does not stop, the performance will end.
So it is with the cells of our bodies and of the birds, insects, animals, and plants whose music fills the Earth. When a jarring note is introduced, no matter how softly, chords become discords, melody becomes noise, life degrades and disappears, and chronic disease epidemics become commonplace.

Life, Information, and Electricity
The cohesion of life does not come from chemistry. It comes from the Earth, Sun, and stars.
K.H. Li wrote, in his forward to the book Electromagnetic Bio-Information:
“The informational aspect of biological systems characterizes the essential view of life. And this is less reflected by biochemical findings, but rather by a level beyond the domain of chemical reactivity, namely that of electromagnetic fields.” [1]
Nikolai Kositsky, Aljona Nizhelska, and Grigory Ponezha reviewed 40 years of research in Ukraine and Russia and concluded:
“Biological effects [of electromagnetic radiation] depend not on the strength of the energy carried into one or another system, but on the information carried into it.” [2]

W. Grundler and F. Kaiser wrote:
“Living cells exhibit a high degree of information processing and communication… It is clearly demonstrated that a fast oscillating, very weak outer field is influencing the biological reactions of cells… We have to take into account an ‘internal’ oscillator (the cell itself or parts of the cell or of its environment) coupling with the outer field.” [3]

John Zimmerman and Vernon Rogers wrote:
“Electromagnetic bioinformation depends on the ability of organisms to emit, receive, and interpret spatiotemporal patterns of electromagnetic fields.” [4]
Herbert L. König, a student of Winfried Schumann, wrote:
“Electromagnetic forces in general must play a role of an as yet incalculable importance in the information transfer between or to living organisms.” [5]

Ulrich Warnke wrote:
“The communicative form of antennae contact in bees and ants can be registered by an oscillograph. Every time a short contact occurs between the antennae, a signal is generated in the recipient’s electrolyte system in the form of an impulse.” [6]
Günther Becker showed that termites’ gallery building rate was affected by termites’ existence in an adjoining container but not if the wall between them was shielded with a conductive material. “These results indicate that communication among termite groups is based on either electric or electromagnetic fields produced by the insects,” he wrote. [7]

Bernhard Ruth wrote that the growth of plants and animals cannot be explained in terms of chemical reactions because “all chemical reactions occur equally in all directions,” and biological growth is directional. “The existing cells of an organism have to determine when and where a new cell is to be generated by mitosis. This can only be achieved by means of an information transfer which stimulates the required cell to divide, and which is not emitted in all directions homogenously.” [8]
Helmut A. Fischer wrote:
“There is good reason for believing that, in addition to mechanical and chemical forms of communication, there are more biophysical ways of communication… The findings made so far confirm that the biochemical processes in a cell, besides thermic effects, also elicit other electromagnetic signals.” [9]

Igor Jerman wrote:
.
“Coherent electromagnetic oscillations in cells permit ordered intermolecular processes and highly selective attractions between enzymes and substrates. These oscillations… represent an important means of intercellular long-range connection and thus have an important role in maintaining an intercellular order… Neoplasms follow from the fact that some of the cells within the organism escape from the intercellular coherent field and thus from the intercellular order.” [10]
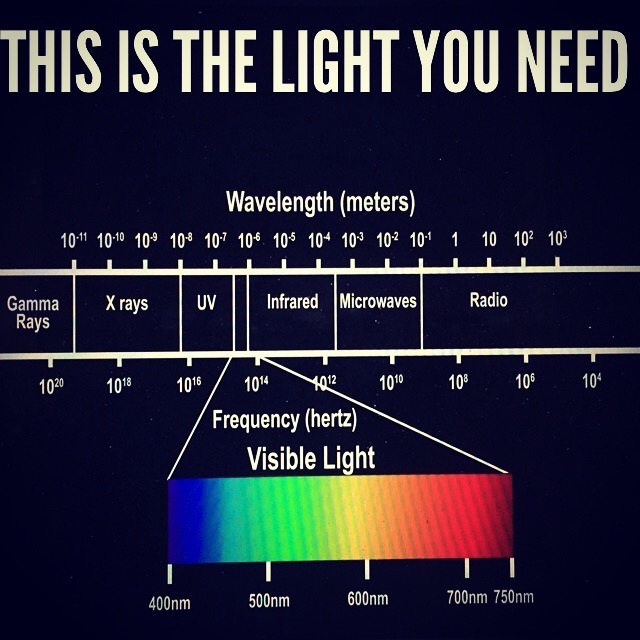
Living cells emit signals throughout the electromagnetic spectrum
In their study, “Electromagnetic emission at micron wavelengths from active nerves,” Allan Fraser and Allan Frey measured infrared emissions from nerves with wavelengths between 2 and 20 microns at a strength of 6 μW/cm2. [11]
Bernhard Ruth detected light photons emitted by plants:
“The light intensity emitted by seedlings of wheat, beans, lentils, and corn varied between 700 cps (counts per second) and 250 cps… The spectral distribution extended from 400 nm to 600 nm… Yeast cells show a radiation of between 150 and 380 nm.” [8]
Shou Sin-Sung wrote that “DNA is a possible radiation source.” [12]

A.H. Jafary-Asl and Cyril W. Smith detected radio frequency signals from yeast at a frequency of 8 MHz. [13]
Herbert A. Pohl detected signals at 7 and 33 kHz from a species of algae. [14]
J. Kent Pollock and Douglas G. Pohl, in dielectrophoresis studies, detected RF emissions from mouse cells at frequencies between 4 and 9 MHz. Similar emissions were detected from cells of bacteria, yeast, worms, chickens, frogs, monkeys, and humans. Maximum emissions occurred during cell division, and no emissions from dead cells:
“The evidence from the m-DEP experiments and from the closely related pattern experiments consistently indicate that cells are producing radio frequency electric fields.” [15]
Sergey Sit’ko and his colleagues measured emissions from the human body between 37-78 GHz at 0.000000000000001 to 0.0000000000000001 μW/cm2Hz. [16]
It takes little or no power to interfere with life

Allan Frey wrote:
“Electromagnetic fields are not a foreign substance to living beings like lead or cyanide. With foreign substances, the greater the dose, the greater the effect — a dose-response relationship. Rather, living beings are electrochemical systems that use very low frequency EMFs in everything from protein folding through cellular communication to nervous system function. To model how EMFs affect living beings, one might compare them to the radio we use to listen to music.
“The EMF signal the radio detects and transduces into the sound of music is almost unmeasurably weak. At the same time, there are, in total, strong EMFs impinging on the radio. We don’t notice the stronger EMF signals because they are not the appropriate frequency or modulation. Thus, they don’t disturb the music we hear. However, if you impose an appropriately tuned EMF or harmonic on the radio, even if it is very weak, it will interfere with the music. Similarly, if you impose a very weak EMF signal on a living being, it has the possibility of interfering with normal function if it is properly tuned. That is the model that much biological data and theory tell us to use, not a toxicological model.” [17]

Gerard Hyland said:
“The human body is an electrochemical instrument of exquisite sensitivity.” [18] “If a signal can operate a mechanical device, it can disturb every cell in the human body.” [19]
Igor Belyaev wrote:
“While the dose rate/SAR concept is adequate for description of acute thermal effects, it is not applicable for chronic exposures to N[on]T[hermal] M[icro]W[aves].” [20] and ”The 51.755 GHz resonance frequency of the cell reaction to MMWs did not depend on power density (PD) in the range from 10(exp-19) to 3 × 10(exp-3) W/cm2.” [21]
Ross Adey, at Loma Linda University, wrote:
“We have discovered some of the keys to understanding how body cells ‘whisper’ to one another, and, in so doing, we have discovered some of the keys to understanding how electromagnetic fields, so weak that some scientists have regarded them as incapable of biological effects, are detected by living tissues, and we have studied some of the likely consequences for human health… These fields can exert effects even at intensities near zero; in other words, a lower limit or threshold may not exist.” [22]
Neil Cherry presented “conclusive evidence” that “the safe level of exposure is zero” [23] and that radio signals “can interfere with hearts, brains and cells at extremely low intensities.” [24]

Dr. Robert O. Becker wrote:
“There is no effective way to shield yourself from environmental fields except to avoid areas where they are prevalent” [25] and “If the system’s sensitivity is as presently described, then frequency becomes a more important parameter in any experiment than field strength.” [26]
In The Body Electric, he wrote:
“The accumulated research has clearly shown that small doses often have the same effects as larger ones… Indeed there has already been one report of brain wave changes suggesting resonance of neural electrical currents with radio waves and microwaves down to a billionth of a microwatt… We must understand that no amount of artificial EMR, no matter how small, has been proven safe for continuous exposure. Bioeffects have been found at the lowest measurable doses.” [27]

Herbert L. König wrote:
“Biological systems have sensitivity values of the same order of magnitude as the field intensity values of natural fields.” [5]
William Bise testified before the U.S. Senate about the effects on brain waves that he elicited by radio waves at near-zero intensity. The results of his experiments ought to terrify every person who ever uses a cell phone and every doctor who is confronted with the extraordinary amount of anxiety and depression in his or her patients today because the radiation in Bise’s experiments, at exposure levels 10,000,000,000 to 100,000,000,000,000 times lower than a cell phone, had strong and instantaneous effects on all subjects’ brain waves and mental states:
“A pilot study was conducted on five men and five women volunteers… They ranged in age from 18 to 48 years. Three had been occupationally exposed to RF energy; the other seven had not, and all were in apparent good health. The RF ranges covered from .1 to 960 Mhz C[ontinuous] W[ave] and 8.5 to 9.6 Ghz pulse modulated. Power levels were varied from 10(exp-16) wt/cm2 to 10(exp-12) wt/cm2… The experimental time for each volunteer was typically 50 minutes…
“Subjects’ EEG traces displayed desynchronized alpha waves of 15 to 25 percent higher than normal amplitude, and slow waves appeared at certain radio frequencies. Conversely, diminution and desynchronization of alpha wave amplitude on the 20 to 50 percent order occurred at other radio frequencies, and 2 to 6 Hz slow waves appeared. These two anomalous patterns were found in both men and women volunteers. Mental attitudes appeared to change during the tests. CW frequencies at a power density of about 10(exp-15) wt/cm2, which produced EEG changes in males, were found between 130 and 780 Mhz. Female volunteers’ EEG alterations occurred between 350 and 960 Mhz. Pulse modulation tests on two males, at a power density of about 10(exp-12) wt/cm2, showed EEG changes around 9.1 and 9.15 GHz. Brain waves changed almost immediately upon tuning a generator to a frequency that produced them and then reverted to their normal patterns when the generator frequency was altered or turned off.” [28] Read Marino’s book “Going Somewhere for more astounding facts you and your doctor are ignorant of.

Sheldon Meyers, Director of the U.S. Environmental Protection Agency’s Office of Radiation Programs, told Congress that “it is not possible to assign a low-intensity limit or threshold below which the exposures are without effect.” [29]
Reba Goodman and Martin Blank wrote:
“Induction of the stress response by magnetic fields occurs at 14 orders of magnitude lower energy density than with thermal stimuli, the current benchmark for cell phone safety standards.” [30]

Yury Shckorbatov found evidence of cell damage after only one second of exposure to 18.75 GHz microwaves at a level of 0.2 mW/cm2. [31]
Low power can be more harmful than high power.
Andrew Wood, Rohan Mate, and Ken Karipidis reviewed 107 experimental studies and found that a lower exposure level tended to have a greater biological effect, and the difference was highly significant (p < 0.001). [32]
Stefano Cucurachi et al. reviewed 113 peer-reviewed field and laboratory studies and found that RF radiation with the lowest power tended to cause the most significant ecological damage. [33]
Maria Sadchikova found that among people occupationally exposed to RF radiation in the 1950s, 1960s, and 1970s, the sickest were those exposed to the lowest, not the highest, levels. [34], [35]
Abraham Lilienfeld analyzed the health of Moscow embassy employees during the 1950s, 1960s and 1970s, when Russia was continuously irradiating the embassy with microwaves. His report was written for the U.S. Department of State. Table 6.32 of his report shows that male employees exposed to the lowest level of radiation had the most symptoms in 18 of 20 symptom categories. [36]

They had more:
depression
migraine
lassitude
irritability
nervous disorders
anxiety
vibrations
intraocular pain
sensations
loss of appetite
difficulty concentrating
memory loss
dizziness
finger tremor
hallucinations
insomnia
neurosis
other symptoms
Liliya M. Fatkhoutdinova studied the effects of video display terminals on blood pressure. Lower levels of electromagnetic fields raised blood pressure more than higher levels. [37]
Vladimir N. Binhi and Robert J. Goldman studied the proliferation of wound cells in response to electric fields. They wrote:
“Most dramatic is that relatively intense electric fields sometimes do not cause appreciable effect while smaller fields do.” [38]

Herbert L. König wrote:
“Exceptionally intense fields often cause no reaction at all.” [5]
Leif Salford, Bertil Persson, Arne Brun, Henrietta Nittby and their team at Lund University in Sweden researched the effects of RF radiation on the blood-brain barrier for 20 years. They found that the lowest levels of exposure caused the most damage to the blood-brain barrier. [39] They calculated that you will do more damage to your brain if you hold a cell phone one meter away from you than if you hold it up to your head. [40]
Dimitris Panagopoulos found that RF radiation reduced reproduction in fruit flies. The maximum impact on fruit fly reproduction occurred when the source of radiation was at some distance away from the flies. [41]
Igor Belyaev, experimenting on E. coli, found that genetic effects occurred at specific frequencies and that the magnitude of the effect did not change with power level over 16 orders of magnitude, all the way down to 0.000000000001 μW/cm2. [21]
Numerous scientists in many laboratories — Carl Blackman et al. at the U.S. Environmental Protection Agency [42]; Suzanne M. Bawin, Leonard K. Kaczmarek and W. Ross Adey [43]; Sisir K. Dutta et al. [44]; Jean-Louis Schwartz, Dennis E. House and Geoffrey A. R. Mealing [45]; and Kumud K. Kunjilwar and Jitendra Behari [46] — found that calcium depletion from brain and heart cells occurred at specific frequencies and exposure levels and did not increase with power. Dutta found a 3,000-fold decrease in power caused a 4-fold increase in calcium exiting from cells.
W. Grundler and F. Kaiser halved the growth rate of yeast at a precise microwave frequency. The magnitude of the effect of this frequency did not change with intensity over several orders of magnitude, down to 5 pW/cm2. [3]
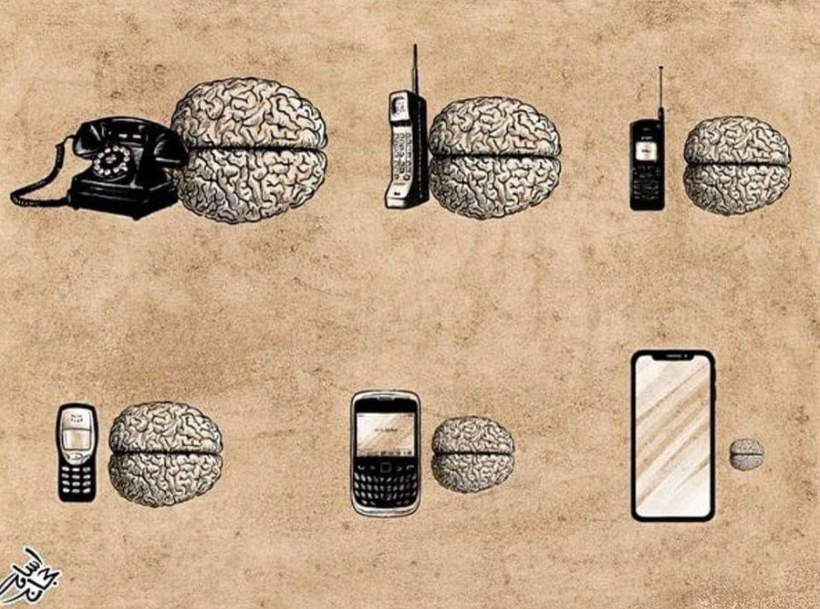
Cooking Your Brain and Your DNA
Here are some other findings that should terrify anyone who uses a cell phone, considering the unprecedented numbers of young people today with cancers and neurological diseases.
First are some measurements made by Markus Antonietti, Director of the Max Planck Institute of Colloids and Interfaces in Germany. In 2006, when cell phone use was becoming universal, he wondered what they might be doing to the brain. Cell phones exposed the brain to about 1 W/kg SAR, which did not heat the whole brain by more than one degree Celsius, but what about the conditions that exist in the tiny synapses, the junctions between neurons where nerve impulses are transmitted from one nerve cell to another? His research team decided to simulate the conditions between cell membranes with tiny fat droplets in salt water. [47] “Ions accumulate on these,” reported Zeit Online, the newspaper that interviewed him, “and by changing the salt concentration and the droplet size, the conditions of biological tissue can be simulated, i.e., a kind of concentrated liquid brain.
“‘And now comes the tragedy,’ said the Max Planck Director. ‘Exactly where we are closest to the conditions in the brain, we see the strongest heating.’ Temperature peaks of 100 degrees. He had expected to warm, but not to this extent. ‘There is a hundred times as much energy absorbed as previously thought. That is a horror.’” [48]
It turns out a cell phone not only boils your synapses but also your DNA. A number of research teams have discovered that DNA is a good conductor and so, as in the synapses, RF radiation is conducted and amplified tremendously in DNA.
Jacqueline K. Barton and her colleagues at the California Institute of Technology in Pasadena observed ultrafast electron transfer in DNA over large distances. [49] “In effect,” she told Science News, “DNA acts like a molecular wire.” [50]

Hans-Werner Fink and Christian Schönenberger reported that the conductivity of DNA is 105 Siemens per meter, which is ten times larger than that of most electrically conducting polymers and about one-tenth the conductivity of mercury. [51]
Charles Polk tells us what the consequences of this are. Based on Fink and Schönenberger’s measurements, Polk calculated that the rate of temperature increase in the interior of DNA exposed to a cell phone at 1 W/kg SAR is 60 degrees Celsius per second! [52].
Your cell phone, if you still use one, is cooking your brain and damaging it, during every second that you use it. The cell towers that it commands are sickening us, no matter how far away from one we manage to be. The satellites — 9,500 of them and increasing rapidly — are polluting our bodies, sterilizing our planet, and severing our connection to our sources of vitality beneath our feet, in the air, in the oceans, and in the heavens.

In my constitutional amendment, there is an entire section dedicated to nnEMF protection that will begin in El Salvador. It not only protects patients but it also has specifics in it for clinicians, students, teachers, and schools. The world is changing right around you now.
Do you sense it yet? If not, why not? Might it be the nnEMF you are imbibing?

CITES
[1] K.H. Li. Foreword to Electromagnetic Bio-Information, Fritz Albert Popp et al., eds., Urban & Schwarzenberg, München (1989).
[2] Nikolai Kositsky, Alona Nizhelska, and Grigory Ponezha. Influence of High-frequency Electromagnetic Radiation at Non-thermal Intensities on the Human Body. No Place To Hide 3(1) Supplement (2001).
[3] W. Grundler and F. Kaiser. Experimental evidence for coherent excitations correlated with cell growth.” Nanobiology 1:163-176 (1992).
[4] John Zimmerman and Vernon Rogers. Biomagnetic Fields as External Evidence of Electromagnetic Bioinformation. In Electromagnetic Bio-Information, Fritz Albert Popp et al., eds., 1989, pp. 226-237.
[5] Herbert L. König. Bioinformation – Electrophysical Aspects. In Electromagnetic Bio-Information. Proceedings of the Symposium, Marburg, September 5, 1977, Fritz Albert Popp et al., eds. Urban and Schwarzenberg, München 1979, pp. 25-54.
[6] Ulrich Warnke. Information Transmission by Means of Electrical Biofields. In Electromagnetic Bio-Information, Fritz Albert Popp et al., eds., 1979, pp. 55-79.
[7] Günther Becker. Communication between Termites by Means of Biofields and the Influence of Magnetic and Electric Fields on Termites. In Electromagnetic Bio-Information, Fritz Albert Popp et al., eds., 1979, pp. 95-106.
[8] Bernhard Ruth. Experimental Investigations on Ultraweak Photon Emission. In Electromagnetic Bio-Information, Fritz Albert Popp et al., eds., 1979, pp. 107-121.
[9] Helmut A. Fischer. Photons as Transmitter for Intra- and Intercellular Biological and Biochemical Communication – The Construction of a Hypothesis. In Electromagnetic Bio-Information, Fritz Albert Popp et al., eds., 1979, pp. 175-180.
[10] Igor Jerman. Electromagnetic Origin of Life. Electro- and Magnetobiology 17(3): 401-413 (1998)
[11] Allan Fraser and Allan H. Frey. Electromagnetic emission at micron wavelengths from active nerves. Biophysical Journal 8: 731-734 (1968).
[12] Shou Sin-Sung. A Possible Biophotochemical Mechanism for Cell Communication. In Electromagnetic Bio-Information, Fritz Albert Popp et al., eds., 1979, pp. 151-174.
[13] A.H. Jafary-Asl AH and Cyril W. Smith. Biological dielectric in electric and magnetic fields. IEEE Annual Report Conference on Electrical Insulation and Dielectric Phenomena, 1983, p. 350.
[14] H.A. Pohl. AC field effects of and by living cells. In Chiabrera A. et al., eds., Interactions between electromagnetic fields and cells, NATO ASI Series A, Life Sciences, Plenum, NY (1985), pp. 435-456.
[15] J. Kent Pollock and Douglas G. Pohl. Emission of radiation by active cells. In Biological Coherence and Response to External Stimuli, Herbert Fröhlich, ed., Springer Verlag, Berlin, 1988, pp. 139-147.
[16] Sergei P. Sit’ko, Yurij A. Skripnik and Aleksey F. Yanenko. Experimental Study of Mm-Range Radiation from Certain Objects. Physics of the Alive 6(1): 15-18 (1998).
[17] Allan H. Frey. Is a toxicology model appropriate as a guide for biological research with electromagnetic fields? Journal of Bioelectricity 9(2):233-234 (1990).
[18] Gerard J. Hyland. Physics and biology of mobile telephony. The Lancet 356:1833-1836 (2000).
[19] Personal communication, December 2018.
[20] Igor Y. Belyaev. Duration of Exposure and Dose in Assessing Nonthermal Biological Effects of Microwaves. In Dosimetry in Bioelectromagnetics, CRC Press 2017, pp. 171-184.
[21] Igor Y. Belyaev et al. Resonance effect of millimeter waves in the power range from 10–19 to 3 x 10–3 W/cm2 on Escherichia coli cells at different concentrations. Bioelectromagnetics 17: 312-321 (1996).
[22] W. Ross Adey. Testimony before the Ad Hoc Subcommittee on Consumer and Environmental Issues of the Committee on Governmental Affairs, United States Senate, August 10, 1992.
[23] Neil Cherry. Evidence of brain cancer from occupational exposure to pulsed microwaves from a police radar. Lincoln University, August 15, 2001.
[24] Neil Cherry. Safe Exposure Levels. Lincoln University, April 25, 2000.
[25} Robert O. Becker. Personal communication, May 15, 1986.
[26] Robert O. Becker. A theory of the interaction between DC and ELF electromagnetic fields and living organisms. Journal of Bioelectricity 4(1):133-140 (1985).
[27] Robert O. Becker and Gary Selden. The Body Electric: Electromagnetism and the Foundation of Life, NY: William Morrow 1985, pp. 312-313.
[28] William Bise. Hearings before the Committee on Commerce, Science, and Transportation, United States Senate, Ninety-Fifth Congress. First Session on Oversight of Radiation Health and Safety, June 16, 17, 27, 28, and 29, 1977, Serial No. 95-49, pp. 1220-1223.
[29] Sheldon Meyers. Oversight Hearing before the Subcommittee on Water and Power Resources of the Committee on Interior and Insular Affairs, House of Representatives, First Session on Health Effects of Transmission Lines, October 6, 1987, Serial No. 100-22, p. 166.
[30] Reba Goodman and Martin Blank. Magnetic field-induced stress responses in biological cells by use of cell phones. EBEA 2001. 5th International Congress of the European BioElectromagnetics Association (EBEA). 6-8 September 2001, Helsinki, Finland. Proceedings, pp. 197-198.
[31] Yury G. Shckorbatov et al., Modification of electrokinetic properties of nuclei and membrane permeability in human buccal epithelial cells under the influence of low-level microwave radiation. EBEA 2001, pp. 204-206.
[32] Andrew Wood, Rohan Mate, and Ken Karipidis. Meta-analysis of in vitro and in vivo studies of the biological effects of low-level millimeter waves. Journal of Exposure Science & Environmental Epidemiology 31: 606–613 (2021).
[33] Stefano Cucurachi et al. A review of the ecological effects of radiofrequency electromagnetic fields (RF-EMF). Environment International 51: 116-140 (2013), Table 4.
[34] Maria N. Sadchikova. State of the nervous system under the influence of UHF. In The Biological Action of Ultrahigh Frequencies, A. A. Letavet and Z. V. Gordon, eds., Academy of Medical Sciences, Moscow, 1960, pp. 25-29.
[35] Maria N. Sadchikova. Clinical manifestations of reactions to microwave irradiation in various occupational groups. In Biologic Effects and Health Hazards of Microwave Radiation: Proceedings of an International Symposium, Warsaw, 15-18 October 1973, P. Czerski et al., eds., 1974, pp. 261-267.
[36] Abraham Lilienfeld. Evaluation of Health Status of Foreign Service and Other Employees from Selected Eastern European Posts. Johns Hopkins University, Department of Epidemiology, Baltimore, MD, prepared for Dept. of State, DC Office of Medical Services, U.S. Dept. of Commerce, National Technical Information Service, July 31, 1978.
[37] Liliya M. Fatkhoutdinova. Hemodynamic indices in VDT users with different exposure to electric and magnetic fields and controls,” EBEA 2001, pp. 292-294.
[38] Vladimir N. Binhi and Robert J. Goldman. The Ion Interference and Electric Field-Induced Wound-Cell Proliferation. In BEMS Twenty-Second Annual Meeting in Cooperation with the European Bioelectromagnetics Association, Abstract Book. The Technical University, Munich, Germany, June 11-16, 2000, pp. 11-12.
[39] Bertil Persson, Leif Salford, and Arne Brun. Blood-brain barrier permeability in rats exposed to electromagnetic fields used in wireless communications. Wireless Networks 3:455-461 (1997).
[40] Henrietta Nittby, Gustav Grafström, Jacob L. Eberhardt et al. Radiofrequency and Extremely Low-Frequency Electromagnetic Field Effects on the Blood-Brain Barrier. Electromagnetic Biology and Medicine 27: 103-126 (2008).
[41] Dimitris J. Panagopoulos. Analyzing the health impacts of modern telecommunications microwaves. In L. V. Berhardt, ed., Advances in Medicine and Biology, vol. 17, Nova Science Publishers 2011, chapter 1.
[42] Carl F. Blackman et al. Induction of calcium-ion efflux from brain tissue by radiofrequency radiation. Bioelectromagnetics 1:35-43 (1980).
[43] Suzanne M. Bawin, Leonard K. Kaczmarek and W. Ross Adey. Effects of modulated VHF fields on the central nervous system. Annals of the New York Academy of Sciences 247: 74-80 (1970).
[44] Sisir K. Dutta et al. Microwave radiation-induced calcium ion flux from human neuroblastoma cells: dependence on depth of amplitude modulation and exposure time. In Biological Effects of Electropollution, Sisir K. Dutta and Richard M. Millis, eds. Information Ventures, Phila., 1986, pp. 63-69.
[45] Jean-Louis Schwartz, Dennis E. House, and Geoffrey A. R. Mealing. Exposure of frog hearts to CW or amplitude-modulated VHF fields: selective efflux of calcium ions at 16 Hz. Bioelectromagnetics 11: 349-358 (1990).
[46] Kumud K. Kunjilwar and Jitendra Behari. Effect of amplitude-modulated RF radiation on cholinergic system of developing rats. Brain Research 601:321-324 (1993).
[47] Christian Holtze, R. Sivaramakrishnan, Markus Antonietti, et al. The microwave absorption of emulsions containing aqueous micro- and nanodroplets: A means to optimize microwave heating. Journal of Colloid and Interface Science 302: 651–657 (2006).
[48] Quoted by Max Rauner in Zeit Online, August 21, 2006.
[49] Chaozhi Wan et al. Femtosecond dynamics of DNA-mediated electron transfer. PNAS 96 (11) 6014-6019 (1999).
[50] Corinna Wu. An Electrifying DNA Debate: New evidence explains how DNA conducts charge,” Science News 156(7): 104-106 (1999).
[51] Hans-Werner Fink and Christian Schönenberger. Electrical conduction through DNA molecules. Nature 398: 407-410 (1999).
[52] Charles Polk. Implications of Measured Electrical Conductivity of DNA for Bio-Effects of E.M. Fields. In BEMS Twenty-Second Annual Meeting, 2000, pp. 22-23.