Quantum Biology at the Interface of Light, Metabolism, and Cellular Dynamics: Insights into Retinal Photobiology, Nitrogen Cycles, and Mitochondrial Symbiosis
The core axiom: All energy in life originates from sunlight, captured and transformed through physical processes like photon absorption, electron excitation, and charge separation. This energy isn’t just “fuel” in chemical bonds (as biochemistry textbooks emphasize); it’s dynamically stored and processed in electronic states of matter within cells, such as excited electrons in proteins, membranes, and quantum coherences.
Step 1: Sunlight as the Ultimate Energy Source and Its Storage Beyond Biochemistry
Start with the basics: The sun emits electromagnetic radiation (photons) across spectra (UV, visible, IR). Photosynthesis in plants converts this into chemical energy (e.g., fructose via the Calvin cycle), but only in environments with sufficient UV/IR intensity, tropical regions where high-fructose fruits evolve. This isn’t coincidental; it’s a thermodynamic necessity, as light provides the energy gradient for carbon fixation and sugar synthesis.
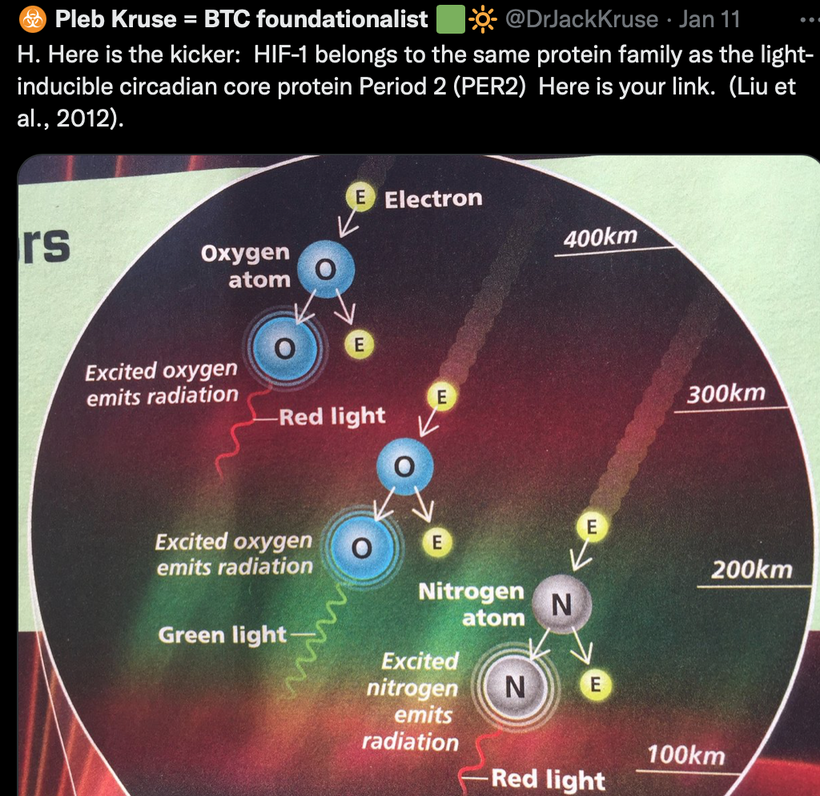
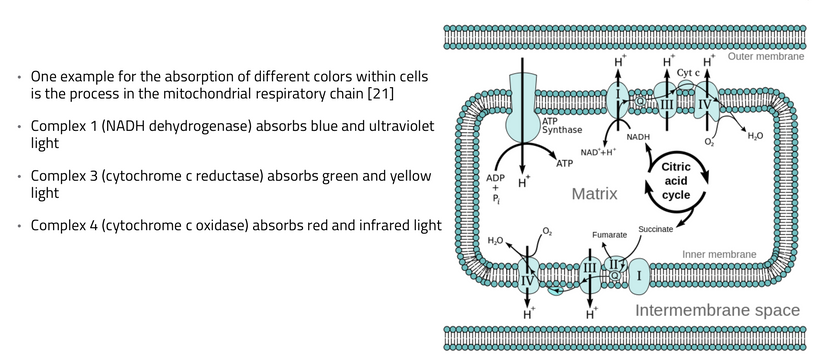
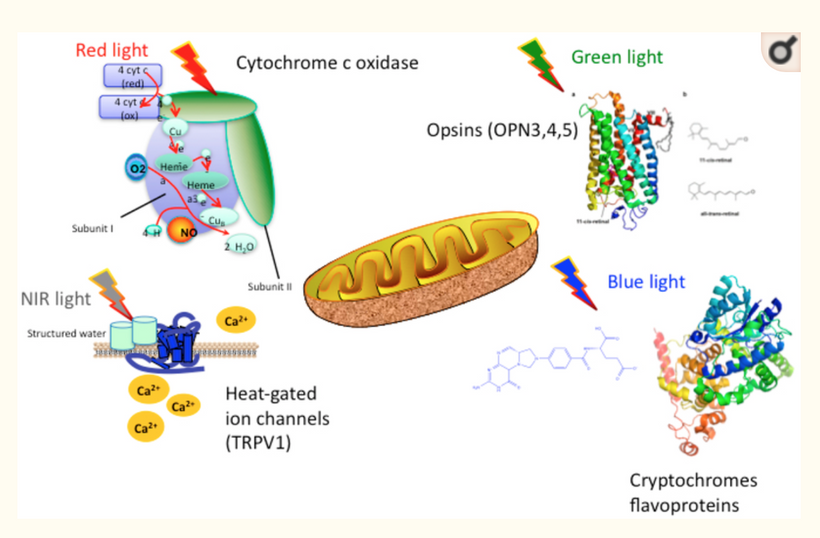
In animals, energy transfer isn’t limited to ingested food. Direct sunlight exposure drives quantum processes: UVA (320–400 nm) releases NO from skin stores, NIR (700–1400 nm) photodissociates NO from mitochondrial enzymes like cytochrome c oxidase (CCO), boosting ATP efficiency. But a new fact based in biophysics expands this, energy is stored in “electronic states” of cells, such as delocalized electrons in aromatic rings (e.g., in porphyrins, melanin) or quantum vibrations in proteins.
These aren’t captured in standard biochemistry books, which focuses on ATP/NADH; instead, they’re quantum phenomena like electron tunneling or coherence, where energy persists in excited states without immediate dissipation.
Why does this matter? It means your biochemistry experts are blinded to the effects of light on biochemistry. Labs measure metabolites (e.g., uric acid levels) but can’t quantify electronic energy storage, things like ultraweak photon emissions (UPEs) from mitochondrial reactions or paramagnetic shifts in melanin. This “biochemical bias” (as in the many images I have shared on amino acid degradation ignoring light) leads to incomplete models. For instance, elevated uric acid isn’t just from fructose phosphorylation depleting ATP; it’s a signal of disrupted energy flow from a solar light deficiency, where electronic states fail to maintain redox balance, slowing TCA/urea cycles.
THE NEW DECENTRALIZED WORLD SEES THESE TARGETS
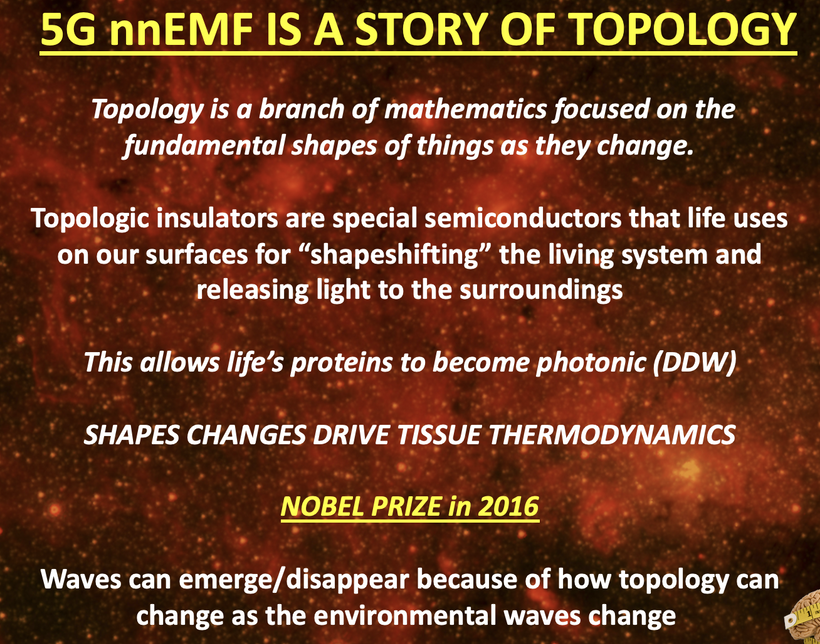
Quantum biology, an emerging interdisciplinary field, explores how quantum mechanical phenomena, such as superposition, tunneling, and entanglement, influence biological processes at the molecular and cellular scales. Laboratories worldwide, from those investigating photosynthetic quantum coherence to mitochondrial electron transport, are uncovering how non-classical effects underpin life’s efficiency and adaptability.
This synthesis on gout (high uric acid levels) integrates classical photobiology with quantum principles, focusing on light’s regulatory role in retinal function, nitrogen metabolism (urea and uric acid cycles), methionine pathways, and melanin-mitochondria interactions.
Drawing from historical studies and recent biophysical research, I propose a “photo-metabolic-quantum axis” where environmental light cues orchestrate mitochondrial redox balance, kinetic efficiencies, and disease susceptibility through interconnected biochemical and quantum mechanisms.

Retinal Photobiology: Light-Induced Damage and Quantum Regeneration Cycles
The retina serves as a prime example of quantum biology in action, where photons interact with molecular systems to drive vision and cellular signaling. A seminal 1971 study (above) demonstrated that diffuse retinal irradiation by visible light induces irreversible damage in rat models, characterized by visual cell death and retinal pigment epithelium disruption.
Exposure to fluorescent cage illumination at 1500 lux through a green filter for 40 hours led to severe retinal degradation, with vitamin A deficiency paradoxically offering protection. This protection arises not from vitamin A acting as a direct toxin upon release from rhodopsin but from disrupting long-range cellular adaptation to light. The study emphasized that the normal diurnal cycle of light and dark is essential for controlling visual cell viability and susceptibility, highlighting quantum-sensitive periodicity in cellular responses.
Contrary to traditional views that the visual cycle regenerates pigments solely in darkness, light-activated enzymes like RGR-opsin in the retinal pigment epithelium challenge this old centralized paradigm. Visible light, particularly green and amber wavelengths, activates RGR-opsin to isomerize all-trans-retinal back to the 11-cis form, enabling daylight-driven regeneration.

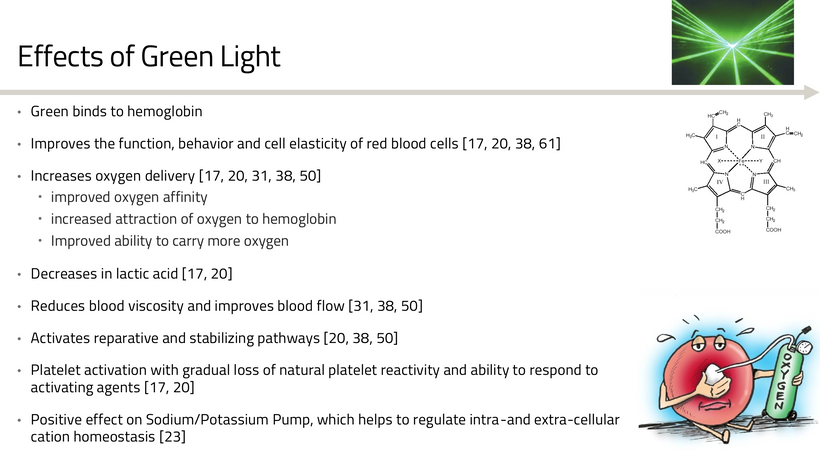
This process underscores quantum principles: photons act as both information carriers and energy sources, with color-specific roles, purple for ignition, blue for tuning (albeit disruptively in excess), green for stabilization of Hb, yellow/amber for synchronization of clocks, and red for photorepair. They all need to be working in unison.
In quantum terms, periodicity functions as an entropy flow meter, sensed by molecular clocks to maintain low-entropy states. Artificial narrow-spectrum LEDs impair this cycle, yielding incomplete repair and elevated entropy, whereas full-spectrum sunlight sustains it, promoting health as a minimized entropy state.
Blue light toxicity, via melanopsin destruction and vitamin A liberation, further indicates low vitamin D3 status, linking retinal quantum events to systemic redox imbalances.
Light Deficiency, Uric Acid, Nitric Oxide, and Mitochondrial Function

Elevated urea or uric acid act as signals for an AM “light deficiency,” reflecting broader mitochondrial slowdowns in the urea and TCA cycles. Few people remember that elevated uric acid inhibits NO. The inhibition of NO in the mitochondria has massive implications for cellular protection and regeneration using light. Normally NO reduces ATP production at the ATPase. NIR light from the sun rescues the ATPase from NO action. What happens if uric acid runs wild and chronically inhibits NO in humans? Remember, NO is the paramagnetic switch in humans that our system uses for activation of stem cells for regeneration using light.

“No morning Sunrise, no Beta-Oxidation” suggests that exposure to morning sunlight is essential for fatty acid oxidation (beta-oxidation), a mitochondrial process that breaks down fats for energy. This is linked to circadian rhythms regulating lipid metabolism via transcription factors like CREBH, PPARα, and FOXO1.

These light factors also control genes involved in fat burning, and disruptions in circadian clocks (e.g., from lack of natural light cues) can impair them. Morning light helps synchronize the body’s internal clock, by enhancing fat oxidation during the day and optimizing the urea cycle; studies show higher fat burning in evenings vs. mornings in some cases, but consistent light exposure supports overall metabolic rhythm.
Since uric acid chronically inhibits NO (by scavenging it or impairing eNOS), and sunlight (UVA/NIR) enhances NO release and dissociation from mitochondrial enzymes like cytochrome c oxidase, then optimal AM light exposure can mitigate uric acid’s downsides, improving mitochondrial kinetics in the TCA and urea cycles. This aligns with quantum biology perspectives where light acts as a “kinetic accelerator” for metabolic pathways. This is another reason why I believe the light environment outweighs dietary inputs in certain environments. Absence of direct comparative trials (light vs. diet) isn’t absence of this effect, because science often lags behind mechanistic insights, especially in underfunded areas like photobiomodulation using IR-A and NIR light.
FRUCTOSE AND URIC ACID
Many clinicians blame fructose toxicity for driving gout. I chuckle at them. Why?
Sun light is required for fructose to exist in foods, because of this causal link in natural solarsettings, its consumption should aligns with light abundance. Today, modern humans have uncoupled this relationship and this is why gout is exploding. No one goes outside enough exposing their liver to sunlight.
Photosynthesis Basics and Fructose Production: Plants capture sunlight (primarily in the photosynthetically active radiation/PAR range of 400–700 nm, but also influenced by UV ~280–400 nm and IR >700 nm) to drive the Calvin-Benson cycle, converting CO2 into simple sugars like glucose, which can isomerize into fructose. Fructose accumulates in fruits as a storage carbohydrate and osmotic regulator of water, enhancing sweetness to attract dispersers (animals/humans). Without adequate solar light energy, the fructose sugar synthesis halts because experiments show low light reduces fructose levels in fruits like tomatoes and strawberries. Most diet gurus forgot the basics of the ecological filter of this aspect of quantum biology.
Environmental Light Conditions for High-Fructose Fruits: Tropical and subtropical fruits (e.g., mangoes, pineapples, bananas—high in fructose) thrive in regions with intense solar radiation: high UV (due to low latitude/altitude), ample IR-A/NIR (which penetrates canopies and aids thermoregulation), and long photoperiods. Studies using LEDs to mimic natural spectra show that red/far-red ratios (simulating sunlight) boost soluble sugars like fructose by 20–50% in fruits, while UV supplementation enhances protective compounds but can modulate sugar balance. In contrast, temperate fruits (lower fructose) grow under milder light regimes. This isn’t speculation; it’s established plant physiology where light intensity and quality directly correlate with fruit sugar content. Robert Lustig and David Perlmuter have a lot of blood on their hands with the fructose advice they have dished out to the public for years.
From a first-principles viewpoint, this implies Lustig and Perlmuter completely missed this evolutionary coupling. In ancestral human environments (e.g., equatorial/tropical origins), consuming fructose-rich foods would naturally coincide with high ambient light exposure. The same solar spectrum that enables plant fructose production (UV/IR-driven photosynthesis) could simultaneously provide humans with UVA-induced NO release and NIR-mediated mitochondrial optimization, offsetting any uric acid elevation from the fructose itself. This creates a balanced “light-diet ecosystem” where light acts as the prime mover, with diet as a downstream proxy. Those who only see biochemistry only see half the story. Modern day gout cases occur in solar deficient humans.
GOUT IS A MARKER FOR A LACK OF UV-NIR LIGHT
I’ll ask you again, what Happens If Uric Acid Runs Wild and Chronically Inhibits NO? Chronic hyperuricemia (e.g., from gout, metabolic syndrome, is a solar “light deficiency”) leading to sustained NO inhibition has cascading effects on vascular, mitochondrial, and regenerative systems. Here’s a breakdown based on evidence: Start with this paper below. PAD is also a UVB deficient disease.

Vascular and Cardiovascular Implications:
- Endothelial Dysfunction and Hypertension: Low NO impairs blood vessel relaxation, raising blood pressure and promoting atherosclerosis. This increases risk for heart disease, stroke, and kidney damage because uric acid is a known CVD biomarker. Reduced NO allows unchecked ROS, fueling chronic inflammation and plaque buildup. This means gout marks when plaques in arteries are increasing in real time. This implies that atherosclerosis, like gout is linked to UV deficiency. Is there any literature on this?

Mitochondrial and Metabolic Slowdowns:
Altered Energy Production: With less NO to regulate CCO, mitochondria might overproduce ATP initially, but chronic low NO disrupts balance, leading to inefficiency, fatigue, and fat accumulation (e.g., hepatic steatosis). High uric acid directly induces mitochondrial ROS and damage, slowing TCA/urea cycles and elevating waste products like ammonia/urea. Gout and PAD are UVDA diseases MAHA will never solve.

Broader Metabolic Issues: Links to insulin resistance, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD), as NO supports glucose uptake and lipid handling.
Hopefully you are beginning to see where all the pieces really fit now.

Light as a Master Regulator of Nitrogen Cycles and Redox Balance
Extending quantum biology to metabolism, light modulates nitrogen handling through the urea cycle, initiated mitochondrially by carbamoyl phosphate synthetase 1 (CPS1) and ornithine transcarbamylase (OTC). While protein intake and mTOR signaling drive the cycle, light’s kinetic influence is underappreciated.
In symbiotic models like giant clams (Tridacna squamosa), light enhances urea absorption and upregulates transporters, potentially via photosynthetic or nitric oxide (NO)-mediated mechanisms. This light-dependent urea active transporter (DUR3-like) boosts nitrogen efficiency, suggesting analogous roles in humans.

Did you know that chronic artificial light at night (ALAN) or blue-dominant exposure mimics hypoxia, inducing oxidative stress and circadian misalignment that slow urea kinetics, risking ammonia accumulation and encephalopathy?

Free ammonia is what causes encephalopathy in man. Remember that red light also reduces glucose levels by 30%. See the above slide.

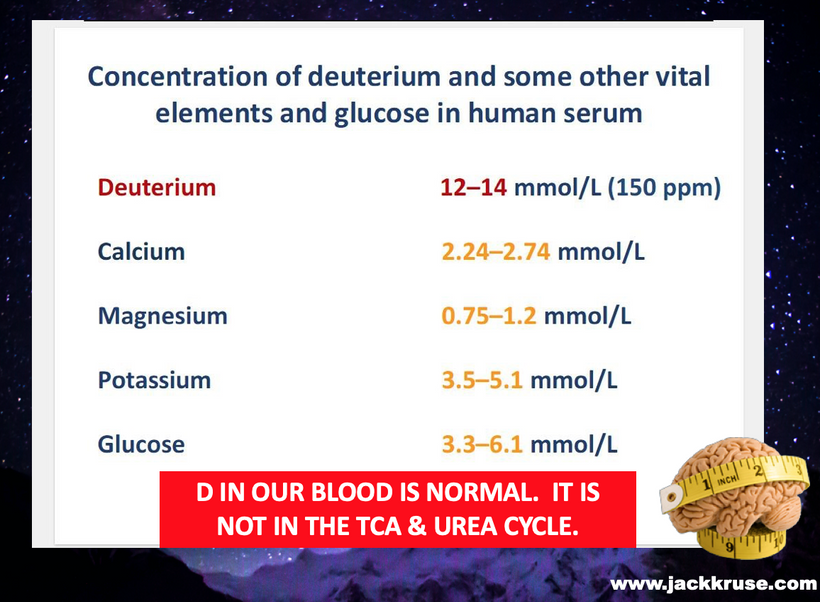
This intersects with uric acid’s inhibition of NO: uric acid reacts directly with NO, depleting it and impairing vascular function. In low-sunlight states, deuterium burden or melanopsin damage exacerbates uric acid buildup, dampening NO and tricarboxylic acid (TCA) cycle flux, fostering a low-redox vicious cycle.
Quantum implications arise in light’s role as a “kinetic accelerator”: morning red/UVA exposure optimizes urea/uric acid disposal, enhancing anaplerosis/cataplerosis. Absent this, mTOR hyperactivation evades DNA repair checkpoints, promoting oncogenesis beyond nutrient sensing, via impaired photorepair of UV lesions.
Vitamin D3/vitamin D receptor (VDR) signaling, diminished by blue toxicity, normally tempers mTOR in sunlit contexts. Thus, AM sunlight restores cycle periodicity, a quantum-sensitive metric of entropy, lowering glucose and uric acid levels reducing gout risks and cancer risk through improved redox and repair dynamics. Gout is a warning that your mitochondria is in worse shape then you or your centralized MD know.
Methionine Metabolism: Sulfur Bridges and Oncogenic Shifts in Quantum Contexts
Methionine’s cascade, from methionine adenosyltransferase (MAT) to S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), homocysteine, cystathionine, cysteine, and propionyl-CoA—links methylation (e.g., for melatonin and carnitine) to TCA entry via succinyl-CoA.
In slowed urea cycles due to poor solar exposure, methionine accumulates, diverting to oncogenic paths: boosting heme/porphyrin for oxygen demand, angiogenesis, and apoptosis inhibition, particularly without UVA/VDR cues. Do you see how this blog now links to reduced RBC mass and anemia now?
Reduced cysteine/glutathione impairs detoxification, allowing toxins to accumulate in low-redox tissues. Uncle Jack is this why nanoplastics show up in the arterial plaques of people who have faulty glutathione and melanin systems in them because they have no skin in the game? Yep.

Solar Light intervenes in this disease cascade: UVA/UVB aids melatonin synthesis, repairing mitochondrial DNA (mtDNA) and restoring kinetics. The mTORC1-c-Myc pathway rewires methionine metabolism in hepatocellular carcinoma progression, dependent on methionine availability.
Deuterium-depleted water (DDW) enhances this by lightening deuterium burden, boosting detoxification indirectly. So yes, I think DDW is another option for those with gout that rarely gets mentioned. Now look back at the slide at the beginning of this blog. Do you see gout is linked to heliotherapy? We’ve know this a long time but BigHarma has made sure your doctors do not learn it. See Lustig and Perlmuter as perfect text book examples of ignorance on this topic. History shows they are both wrong.

Quantum biologically, methionine bridges nitrogen cycles to effects like proton tunneling in enzymatic reactions, where light-deprived states amplify oncogenic potential through disrupted entropy flows.
Melanin-Mitochondria Symbiosis: Paramagnetism, Ultraweak Photon Emission, and Deuterium Dynamics
This part of the blog is for the scientists and clinicians who think I am joking how wrong the paradigm really is on gout.
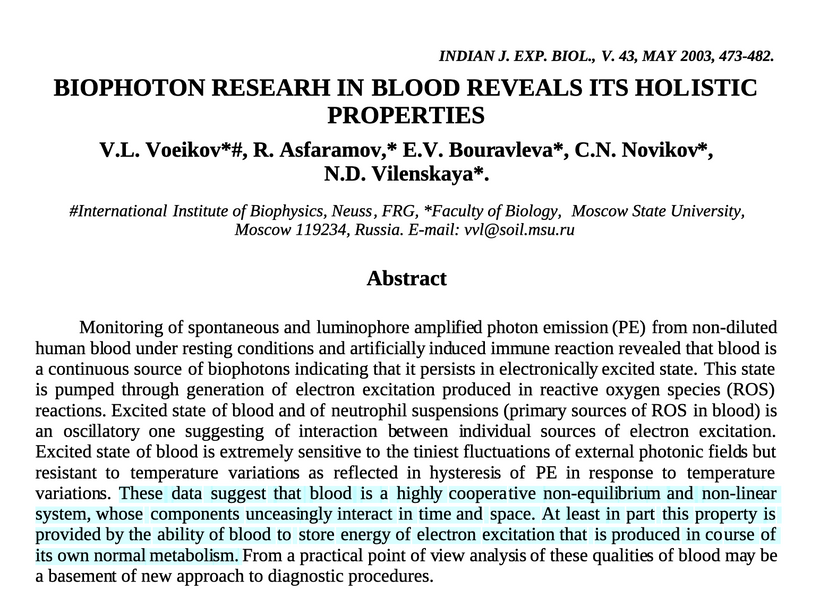
Melanin’s proximity to mitochondria positions it as a quantum sensor, scavenging reactive oxygen/nitrogen species (ROS/RNS) and modulating ultraweak photon emission (UPE)—spontaneous low-level luminescence from biological systems. A slide above mentions TCA/urea cycle dynamics and UPE creation. Review it again. Recall, melanin is also paramagnetic, and that ability is also hydration-dependent! This means CCO is critical in melanin biology. This acts to shift hydroxyquinone-semiquinone equilibria, while tuning proton gradients via interactions with ATP synthase’s magnetic fields from Fo rotation of the ATPase.
DDW amplifies this by enhancing proton mobility (e.g., Zundel ions), stabilizing radicals, and boosting electron paramagnetic resonance (EPR) signals, which we have great data on. Your docs do not know this, but I do, because I read the physics journals who use EPR all the time.
Presently, this is uncharted work in biophysics but aligned with DDW’s mitochondrial tweaks I have used for 20 years now. Melanin absorbs/scatters UPE from mtDNA/ROS, enabling non-chemical mitochondrial communication via UPEs, while paramagnetism of melanin/NO/oxygen optimizes deuterium discrimination in matrix water.
In low-sun states, dehydration or deuterium enrichment weakens this symbiosis, slowing TCA/urea cycles, elevating uric acid, and promoting cancer via low melatonin and unchecked ROS. Melanin-rich tissues (skin, eyes, brain) thus act as light-sensitive redox hubs, with emerging therapies like DDW plus morning sunlight hydrating melanin for UPE-guided repair, countering blue-induced damage.
My Unified Framework on Gout: A Photo-Metabolic-Quantum Axis in Health and Disease
This integrated decentralized view posits uric acid/urea elevations as markers of “light deficiency syndrome,” where absent solar cues disrupt NO-TCA-urea-methionine-melanin loops, amplifying deuterium’s kinetic drag inside the mitochondrial matrix, causing mTOR dysregulation, disrupting wound repair, and lead to epigenetic oncogenic shifts in genes. Gout is a pre malignant marker in my thesis.
Cancer is linked to Chronic Reduced Regeneration: NO is crucial for stem cell proliferation, wound healing, and tissue repair. Nitric oxide is an optical signaling “switch” for angiogenesis and anti-apoptosis. Chronic inhibition of NO slows recovery from any injury, it increases the aging processes via heteroplasmy rate expansion, and leads to chronic diseases like cancer (where NO has dual roles). Cancer manifests as de-synchronized mitochondrial adaptation, hijacking slowed cycles for survival, with paramagnetic melanin failing to “tune” the system properly under the hypoxia of ALAN. This makes even small amounts of oxygen a toxin and this is what drives oncogensis. It fully aligns with my quantum models of mitochondrial redox optimazation.
SUMMARY
Future validation avenues of my work should focus on EPR studies on DDW-hydrated melanin in UVA-exposed models, bridging biophysics gaps. This work needs to be added to Pollack’s old and new work on water and DDW. For quantum biology learners, this framework illustrates how light’s quantum interactions (e.g., photon absorption, UPE) scale to macroscopic health outcomes.
Practical Implications for Quantum-Informed Interventions
To leverage these insights:
- Prioritize sunrise-to-10 AM exposure for red/UVA-driven melanopsin/Vitamin D3/NO cycles rebuilding and cycle acceleration.
- Consume latitude-local, circadian-appropriate foods, emphasizing seafood for low-deuterium fats and sulfated amino acids.
- Avoid ALAN to preserve UPE/melatonin periodicity. Use salt liberally to increase UV assimilation to avoid UVDS diseases.
- Consider DDW to enhance melanin/TCA function, monitoring uric acid as a sunlight/redox biomarker in treating gout.
This paradigm shifts quantum biology from theoretical curiosity to actionable science, urging labs to explore light-metabolism entanglements for novel decentralized therapies that are dirt cheap to implement.
CITES
https://www.linkedin.com/pulse/finding-calling-out-centralized-bullsit-jack-kruse/