If you are following the series, you will see the story of light stress, which was used in an adaptive fashion by mammals 65 million years ago to survive. What underpins this effect, however, may shock you. It is POMC. It turns out that repeated exposure to low levels of mitochondrial stress, which environmental light induces, and various cytosolic and nuclear responses build resilience against higher levels of stress. This response would have significantly benefited mammals during an extinction-level event. This adaptive response, primarily known as mitohormesis, has been shown to extend health span and/or lifespan in several model organisms (Yun and Finkel, 2014).
What are the consequences here?
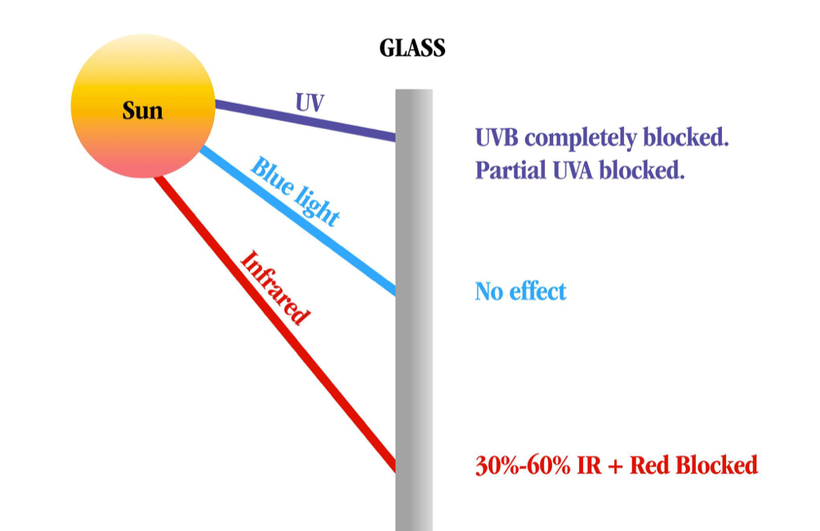
Ancient adaptions can lead to modern diseases when the spectrum of light changes again. Remember, the initial adaptation mammals made was due to a lack of UV light in their environment, and they ran predominantly from 390nm to -3100 nm.
Today, centralized medicine looks at hemochromatosis as a disease when, in reality, it occurred as an epigenetic adaptation for an iron protection strategy that humans used to keep adult males alive to reproduce to allow them to survive in Europe for the last 1000 years. Today, scientists are beginning to realize that our ‘junk DNA’ seems to be the raw material for constructing new wide-bandgap (WBG) semiconductors that use light to sculpt changes. This is how our semiconductors transform light into epigenetic information to change the game so survival is guaranteed. Evolution seems to tell us that in the last 1000 years, biology built a new way to defend against pathogens and events we have recently faced so that we can survive whatever life throws at us. This semiconductive fabrication plant in our bodies (POMC) acts like an evolutionary junkyard that allows us to innovate new novel ways to survive a bad event.
We now know that transgenerational epigenetics in the Viking men of the Northern coastline of Northern Europe was selected for hemochromatosis. We believe that the COLD, harsh Tundra of the north was mineral deficient. Women with this genetic defect would have fared as better child bearers because they could absorb more iron to birth more children who also carried the hemochromatosis defect into the next generation. It is also believed that the Viking men might have survived the disease because their Gladiator-type lifestyle was ferocious, and they often faced severe blood loss that might have offset the iron defect. As Vikings settled in Northern Europe, the mutation grew using the “founders effect” caused by inbreeding due to small population sizes. The founder effect means that any ‘non-lethal defects’ are highly selected for and carried in the entire population of a people. It is believed that the ‘Viking defect’ was blended into the populations of Northern and Western Europe over 500 years to solve the recurrent Yersina outbreaks that caused the bubonic plague and almost extinguished humans in Europe. The chronicity of the infection was an ‘epigenetic signal phenomenon’ that allowed for the selection of the hemochromatosis gene to confer reproductive fitness over longevity. At the same time, the Yersina remained active in the human population. This remained true for close to 500-1000 years.
This “irony” may now explain why ancient physicians were barbers and bloodletters. We used to believe this practice was ‘quackery,’ but we now know that it was the survival of the wisest in action. Bloodletting had a significant role in conferring more longevity to those with hemochromatosis of European descent. When you bleed, you release a massive dose of vasopressin from the posterior pituitary.
Until the 20th century, bloodletting was standard practice. Then, it was stopped, and hemochromatosis became a modern disease. Canadian physiologist Norman Kasting found that bloodletting also released the hormone vasopressin (ADH) from the posterior pituitary. This release from the hypothalamus reduced their fevers and increased their immune function to act faster to save them. This finding was not causation, but the correlation between bloodletting and fever reduction is massive in human history. Bleeding them down may have helped fight infection when it was present. When we bleed, vasopressin release is also altered. nnEMF does the same.
AUTOIMMUNITY AND VASOPRESSIN
Abnormal non-circadian release of vasopressin is linked to autoimmune conditions in modern man.
The arginine vasopressin hormone (AVP) of the posterior pituitary increases blood−brain barrier permeability. It also affects voltage gates and water balance in cells.

The immune system (IS) plays a vital role in protecting our body and can recognize its tissues from foreign molecules. The IS comprises lymphoid organs, cellular components, humoral factors, chemokines, and cytokines that respond to antigens that can harm the body (Shinde and Kurhekar, 2018; Verbsky and Routes, 2018).
The IS is highly and tightly regulated by mtDNA signaling and biophoton biology; however, its disruption could induce diverse pathological disorders, such as allergies and asthma; subsequently, when the host’s tolerance is exceeded, the condition becomes an autoimmune disease (Anaya et al., 2016).
nnEMF exposure is critical to BBB function, as Russ Adey and Allen Frey demonstrated in the 1960s.
Most autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, Hashimoto’s thyroiditis, Crohn’s disease, type 1 diabetes mellitus, and MS are considered chronic illnesses today by centralized medicine. I consider them modern diseases linked to aberrant lighting and nnEMFs.

Some of them can induce neuroinflammatory and neurodegenerative processes in the CNS, which occur when the integrity of the BBB is compromised, as the pictures above show. The BBB is considered a highly selective barrier between the cerebral capillary blood and interstitial fluid of the CNS (Kadry et al., 2020; Schreiner et al., 2022) and helps keep harmful substances from reaching the brain as pathogens, toxins, and some drugs, as well as prevents the entry of IS components (Daneman, 2012; Kadry et al., 2020; Knudsen et al., 2022).
The principal constituent of the BBB is the endothelial cells, which provide protection and structural stability to the blood vessels on tight junctions (the liner sheets pericytes and astrocytes that ensheath the blood vessels and restrict the substances entering the brain or its immune system. The breakdown of the BBB promotes its permeability, permitting the entrance of immune molecules and lymphocytes, inducing autoreactive conditions in the blood-spinal cord barrier and BBB; as a consequence, damage and destruction of the myelin sheath increases, causing neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, ALS, and MS.
Similarly, neuroinflammation promotes the entry of IS components through the BBB and blood-spinal cord barrier into the CNS; therefore, an increase in the barriers’ permeability induces the interaction of pro-inflammatory cytokines such as the interleukins 1β and 17A (IL-1β and IL-17A), and tumor necrosis factor α (TNFα), which activates the downregulation of tight junctions in the endothelial cell barriers. IL-17A has been associated with the loosening of both obstacles, as shown by in vivo and in vitro assays. It is related to the production of reactive oxygen species by nicotinamide adenine dinucleotide phosphate (NADPH) and xanthine oxidase action, which are related to increasing in the endothelial cells’ permeability, causing a decrease in the occluding protein, zonula occludens-1 in in-vitro assays by Arima et al., 2013. The photoswitch I have mentioned before is a critical circadian controller of the ROS mechanism.
In contrast, the hyperactivity of sensitized lymphocytes induces the proliferation and secretion of IL-17 in MS. In addition, under normal conditions, T regulatory (Treg) cells alter and break down the balance in IS responses, which are accompanied by MS (Pot et al., 2011). Immune cells such as T and B lymphocytes, macrophages, and innate immune cells promote the disease’s pathophysiology. Myelin antibodies from B cells induce the loss of the myelin sheath. Furthermore, studies have demonstrated the presence of immunoglobulins IgG and IgM in patients with acute and chronic MS.
THESE DISEASES ARE MULTIFACTORIAL, BUT LIGHT AND POMC DYSREGULATION ALWAYS AT THE CORE
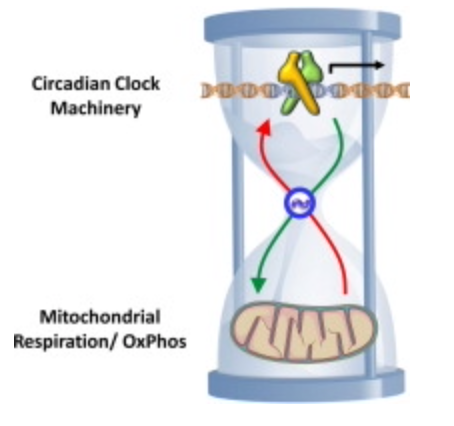
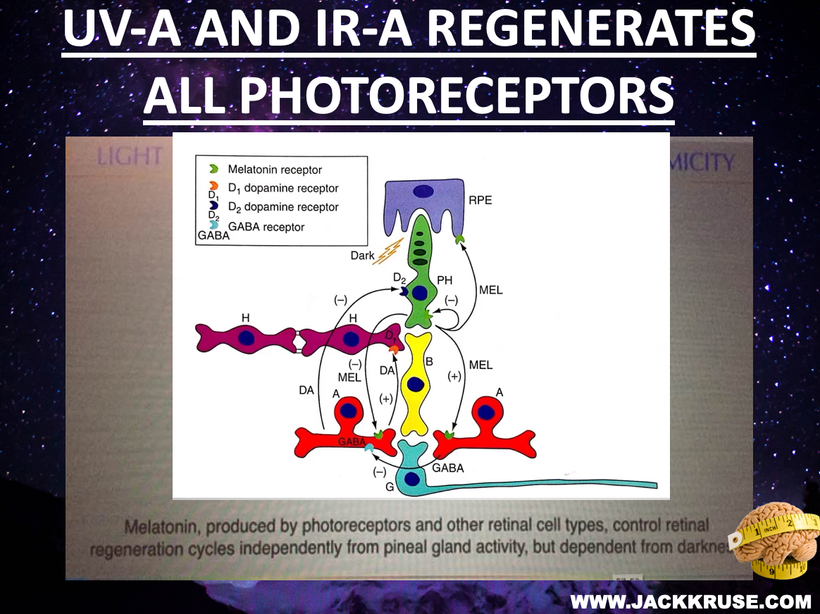
The human hypothalamus is a pivotal governing center for various metabolic processes in the body (Roh et al., 2016; Waterson and Horvath, 2015). Proopiomelanocortin (POMC)-producing neurons in the hypothalamic arcuate nucleus (ARH) are critical in regulating energy and water balance. (below)

POMC neuronal activity is strongly tied to mitochondrial function. The higher your mitochondrial redox power is on your inner mitochondrial membrane, the more POMC a tissue will express. More POMC = more semiconductors can be made. POMC neuronal activity can be upregulated in mice by mitochondrial-derived ROS (Diano et al., 2011). Moreover, mitochondrial dynamics (i.e., fusion and fission) in POMC neurons are essential for maintaining whole-body energy and glucose homeostasis under altered metabolic conditions (Ramírez et al., 2017; Santoro et al., 2017) in all mammals. Despite the evident importance of mitochondria-originated signals in POMC neurons (Mishra et al., 2014), the details of the underlying mechanisms remain largely unknown in centralized medical research.
MITOCHONDRIAC UNDERSTANDING: SUNLIGHT IS MANDATORY for making water at CCO during the day in mammals. If you do not get enough sun or live at a high latitude inside all day, you need more water to avoid the vasopressin release issued POMC. If we do not get enough sunlight, we’re dehydrated, and then we lose circadian feedback control of vasopressin, and the entire water cycle in our body goes awry. This facilitates the development of autoimmunity.
This is how lousy clock management leads to epigenetic disease by decreasing mitochondrial redox power. This is why big pharma is now pushing the use of anti-vasopressin analogs for MS patients. Understanding POMC is understanding modern human neolithic diseases. Mammals are creatures sculpted by light.
Recent work in this area shows that POMC neurons exhibit a dimorphic (biphasic) response to mitoribosomal stress in a dose-dependent manner; homozygous deletion of Crif1 was detrimental (i.e., severe light stress), whereas heterozygous disruption was beneficial (i.e., mild light stress).
How does IR-A light work in the sun? A biphasic dose response has been frequently observed where low levels of red light have a much better effect on stimulating and repairing tissues than higher levels of light. The so-called Arndt-Schulz curve is commonly used to describe this biphasic dose response. Centralized medicine has no idea how POMC works with light. POMC gets cleaved using the biphasic actions in light. When cleavage is imprecise, disease results. Now you do, too.

Published research has found that low levels of mitoribosomal stress in POMC neurons induced high metabolic turnover and resistance to obesity through cell-non-autonomous mitochondrial stress signaling between the hypothalamus and adipose tissues. That stress signal is VUV light emission in hypothalamic neurons in the leptin-melanocortin pathway. That is how you stay thin. It would be best to renovate the melanin sheets inside your tissues constantly.
In humans, prolonged or repeated cold exposure without surface-level UV light can increase the mass and activity of brown adipose tissue in the neck and supraclavicular regions, as defined by the uptake of glucose, and can improve glucose homeostasis. This is the off switch for ACTH release from POMC mammals used to survive in high latitude and poorly lit cold areas.
POMC neurons control adipose tissue thermogenesis (Dodd et al., 2015). I knew 15 years ago that this discovery, as pictured below, was coming. Brown adipose tissue works its job because of melanin. Surprise!

Mitochondrial communication via optical transmission is the key to adaptive stress response following mitochondrial perturbation. Recently, small functional peptides encoded within the mtDNA, known as mitochondrial-derived peptides (MDPs), have been identified (Reynolds et al., 2020). MDPs represent a unique class of mitochondrial-encoded signaling factors that respond to mitochondrial stress and promote health/longevity (Galluzzi et al., 2018; Mottis et al., 2019; Quirós et al., 2016; Tan and Finkel, 2020).
Notably, MOTS-c (mitochondrial open reading frame of the 12S rRNA-c) mediates mitonuclear communication by translocating to the nucleus upon metabolic stress and regulating adaptive nuclear gene expression to promote cellular homeostasis (Kim et al., 2018).
Light sculpts your life. VUV light, to be exact, when it comes to autoimmune disease conversion. That tells me that mtDNA biophoton release is where the defect is because terrestrial sunlight does not contain these frequencies.
Vasopressin (AVP) is a crucial hormone regulating water balance and is released during hyperosmolality to limit renal excretion. It has a long history to explore. It is produced by adjacent melanin in humans. Arginine-vasopressin (AVP) is a nonapeptide that is synthesized mainly in the supraoptic (SON), paraventricular (PVN), and suprachiasmatic nucleus of the hypothalamus. In addition, AVP is produced in several other brain areas and organs, e.g., the medial amygdala, bed nucleus of stria terminalis (BNST), and the adrenal gland chromaffin cells. Its release is associated with any body stress response, opening the blood barriers to the gut and brain.
SUMMARY
When vasopressin is altered, so is iron biology, which leads to an altered amount of ROS/RNS. Why?
Re-read this blog. It tells you how light and ROS are linked. https://www.patreon.com/posts/quantum-75-p53-106441242
Why do we say oxygen is “reduced” when iron is oxidized in our body?
We say this because iron has gone from its elemental state with no charge ( Fe0) to its ionic state (Fe3+). Because the iron has lost electrons and become positively charged, it has been oxidized. The oxygen has been reduced because it gained the electrons iron donated to it. The electrons from the iron went to the oxygen. Every oxidation process has to have a corresponding reduction.
Iron exists predominantly in two biologically relevant redox states: ferric iron, the oxidized state (Fe3+), and ferrous iron, the reduced state (Fe2+). Fe2+ is well known to facilitate electron transfer reactions that can lead to the generation of reactive oxygen species.
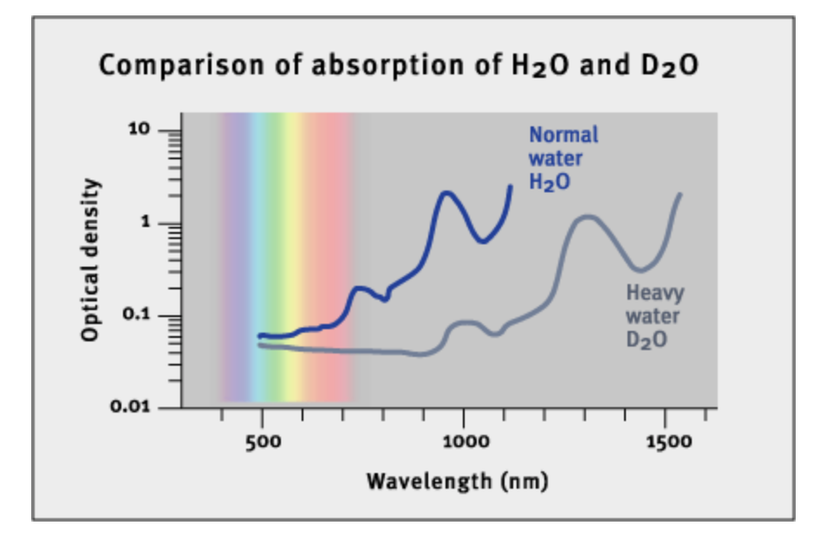
The involvement of singlet oxygen in biophoton emission has implications for our understanding of many diseases like mast cell disease in the skin that links to immune function. Mast cell dysfunction is related to an absence of 1270 nm light in the skin of mammals. Singlet oxygen is known to liberate this frequency of light, as the picture in this blog shows. People with mast cell disorders do not make enough hydrogen peroxide from their mitochondrial respiration. As a result, a comorbid lack of sunlight containing 1270 nm light and lack of H202 creation in tissues is associated with immune dysfunction in mast cells. There is a lesson here that the photoswitch in our cells is teaching us about immune dysregulation.
People with autoimmunity, mastocytosis, and poor wound healing are always deficient in AM sunlight. Why? This is when we get a lot of NIR light with 1270 nm. Early morning sunlight, 6 AM -9 AM, has a relative irradiance with a higher amount of photons in the visible and NIR spectrum than midday exposure (noon). The picture tells why decentralized medicine always recommends AM sunlight. This sun time = TINA = THERE IS NO ALTERNATIVE.

CITES
G.T. Dodd, S. Decherf, K. Loh, S.E. Simonds, F. Wiede, E. Balland, T.L. Merry, H.Münzberg, Z.Y. Zhang, B.B. Kahn, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat.
Alvarez, J. I., Cayrol, R., and Prat, A. (2011). Disruption of central nervous system barriers in multiple sclerosis. Biochim. Biophys. Acta BBA – Mol. Basis Dis. 1812, 252–264. doi: 10.1016/j.bbadis.2010.06.017
Anaya, J.-M., Ramirez-Santana, C., Alzate, M. A., Molano-Gonzalez, N., and Rojas-Villarraga, A. (2016). The autoimmune ecology. Front. Immunol. 7:139. doi: 10.3389/fimmu.2016.00139
Arima, Y., Kamimura, D., Sabharwal, L., Yamada, M., Bando, H., Ogura, H., et al. (2013). Regulation of immune cell infiltration into the CNS by regional neural inputs explained by the gate theory. Med. Inflamm. 2013:898165. doi: 10.1155/2013/898165
ASTELLAS Pharma US, Inc. (2005). Vaprisol (conivaptan hydrochloride) injection. Rockville, MD: Department of Health and Human Services. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021697s000_Vaprisol_Approv.pdf
Baron, J. L., Madri, J. A., Ruddle, N. H., Hashim, G., and Janeway, C. A. (1993). Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J. Exp. Med. 177, 57–68. doi: 10.1084/jem.177.1.57