Recall that sunlight is 42% infrared and this is nature’s version of photodynamic therapy. UV/IR combo light in sunlight is your vaccine to cancer. Amongst all the different types of cancer treatment, photodynamic therapy (PDT)- where light in is used to destroy malignant cells – might have one of the strangest side effects: patients are often better able to see in the dark. Why? with PDT red light is used to lyse cancer cells. The same red light stimulates the repair of the photoreceptors in the eye and affects how retinal acts with those photoreceptors.
Normally in the retina, the combo of UV/IR light from the sun regenerate the photoreceptors in the eye to optimize the function of ipRGC’s and many other photoreceptors in the central retinal pathway. This pathway also controls the neurohormone response in humans and that is why PDT and the sun can be used to treat different cancers.
For example, the intrinsically photosensitive retinal ganglion cells (ipRGCs) exhibit several important functions including circadian photoentrainment, pupillary light reflex, alertness, and phototaxis. We now have definitive evidence that ipRGCs regulate other physiological activities via the autonomic nervous system. These autonomic ganglia are highly dependent upon the TCA cycle and thiamine dynamics to work optimally.
The sympathetic system uses acetylcholine at the proximal ganglion and norepinephrine at the distal terminal ganglion.
The parasympathetic, or craniosacral outflow, opposes the actions of the sympathetic system. It uses acetylcholine at both the proximal and distal ganglia. Increased tone in one system is modulated by the decreased tone in the other, an essential balance that results in a continuous adaptation to the environment. The concept of homeostasis is more aptly seen as homeodynamics, a continuous reaction between the environment and the adaptive status of the organism.
It has been shown in the literature that external light stimulation can activate hair follicle stem cells through the central retinal pathways semiconductive circuits via an ipRGC–suprachiasmatic nucleus–sympathetic nervous circuit. Immediately after ipRGCs are stimulated by light, the systemic sympathetic activities are activated in the brain stem that synapse in ganglia that innervate the skin. In the skin, the local release of the biogenic amine norepinephrine (made of tyrosine another aromatic amino acid) which is the key stimulus that activates hair follicle stem cells to regenerate and grow hair. This neural circuit in the retina enables prompt communication between peripheral tissues and the external environment just using light via the retina. This tells us wearing glasses, contacts, or sunglasses has to affect the physiology of the system. It also is really bad news for those who have permanently implanted intraocular lenses. Due to the systemic activation of sympathetic activities, this circuit can also allow for timely responses to external light in other organs in the body to perform many autonomic tasks. This highlights how a 5G topologic effect on the eye or skin can lead to chronic thiamine deficiency and the development of autonomic instability associated with EHS or POTS. It also highlights a function of ipRGCs in regulating autonomic nervous activity in the body.

The sympathetic nervous system plays an important role in the regulation of adipose tissue lipolysis. This is why blue light fattens humans and obese humans and diabetics often have evidence of thiamine deficiency when we look for it.
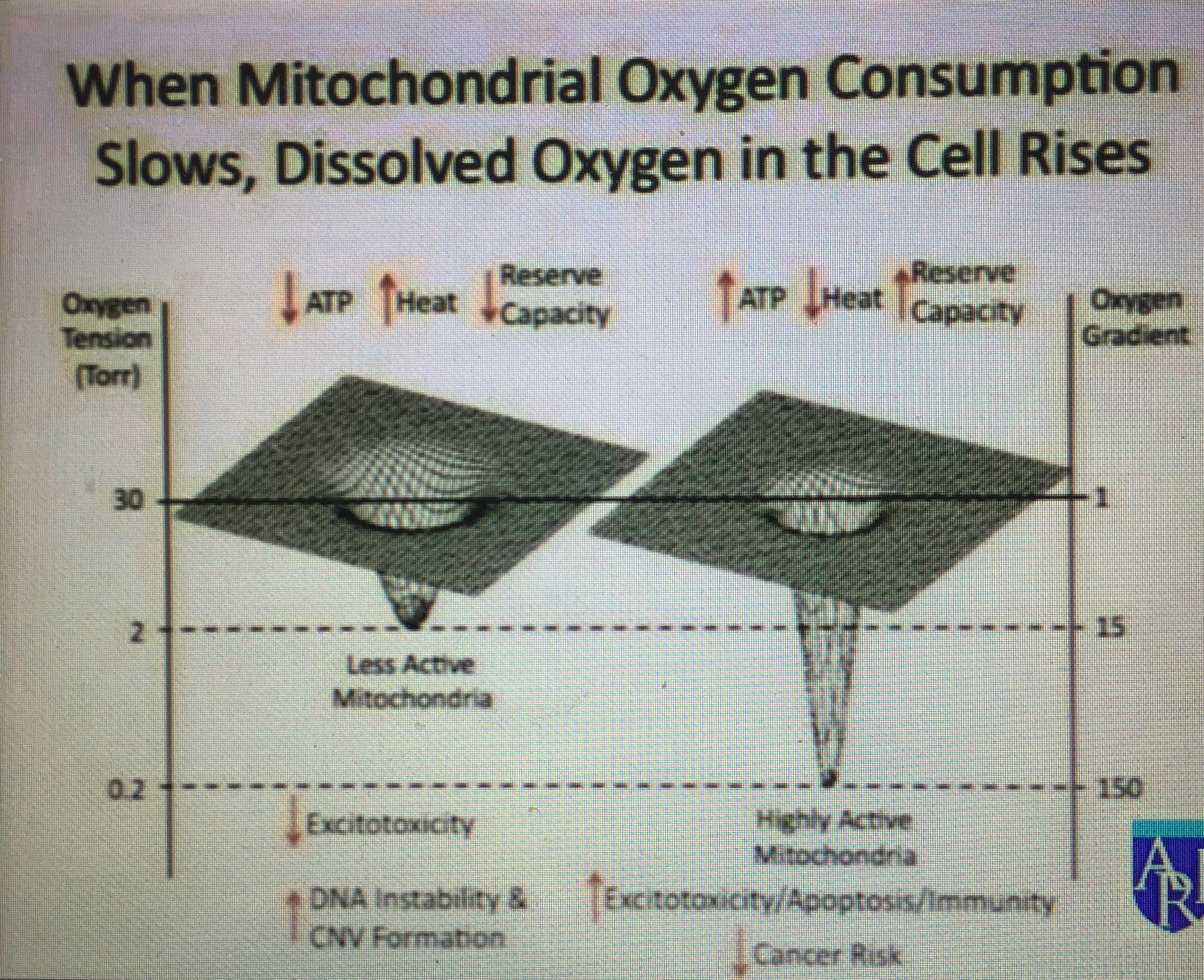
Chronic exposure to moderate and severe hypoxia increases the activity of the sympathetic nervous system and adrenal medulla. This is associated with chronic thiamine deficiency and low levels of Vitamin A and high level retinol binding protein in the blood. These are all markers of nnEMF/blue light toxicity and thiamine deficiency. Vitamin A is a retinal derivative in humans. Blue light and nnEMF alter Vitamin A levels and thiamine levels to cause hypoxia and lowered levels of oxygen consumption at the inner mitochondrial membrane. It also slows the turn rate of the TCA cycle and lowers ATP formation at the ATP synthase.

One of the important observations made in studying patients with thiamine deficiency is that arterial oxygen saturation in the regions of the body innervated by the sympathetic ganglia is relatively low in the disease when venous oxygen concentration is relatively high. This tells us that the mitochondria in that region are not extracting oxygen well because of altered TCA dynamics due to the lack of thiamine. Blue light and nnEMF decrease thiamine stores. The same phenomenon has been reported in sleep apnea and has been reported numerous times in the literature to be related to glucose dysregulation.

What explains humans’ ability to improve night time vision with IR-A light? Does this somehow affect melanopsin and thiamine metabolism?
The answer is yes.
Recently, researchers have figured out why red light therapy during cancer treatment improves night time vision and can act as a chemotherapeutic agent against cancer: rhodopsin, a light-sensitive protein in the retinas in our eyes for night vision, interacts with a photosensitive compound called chlorin e6, a crucial component of this type of cancer treatment.
The work builds on what scientists already know about the organic compound retinal, which is found in the eye and usually isn’t sensitive to infrared light. It is very sensitive to blue light frequencies. When this chemical is liberated it is known to cause photoreceptor damage.
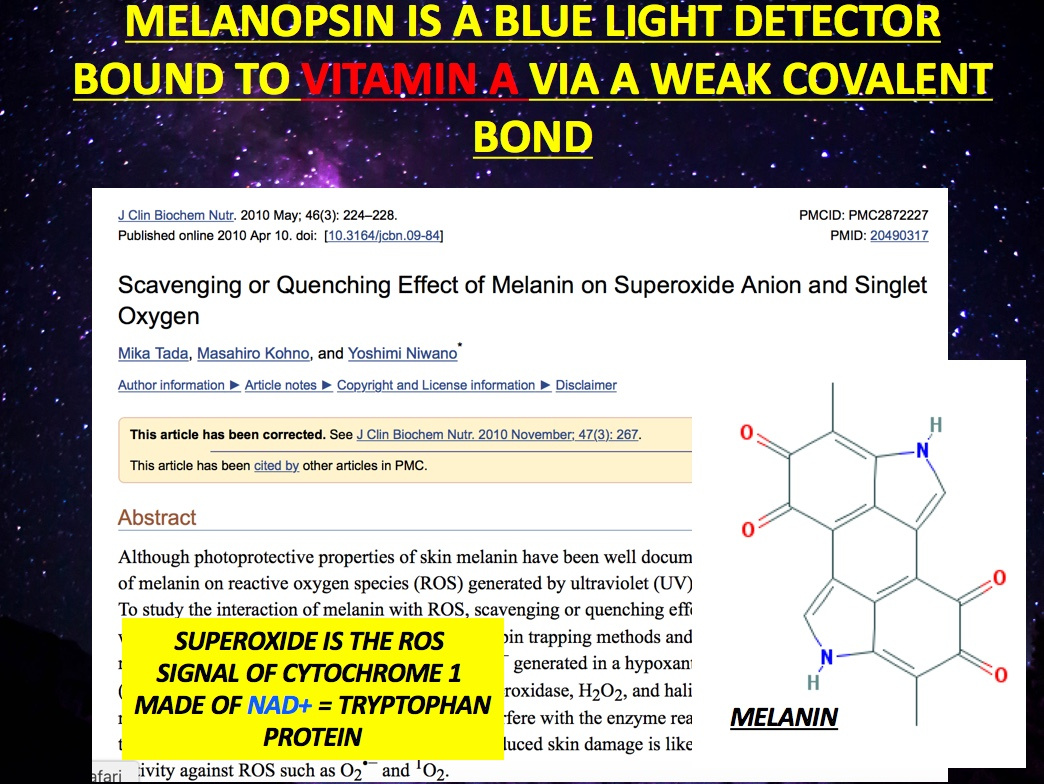
Visible light triggers retinal to separate from rhodopsin. Blue light causes retinal to separate from melanopsin due to its weak covalent bond. IR-A light does not have enough power to do this. The addition of chlorin e6 makes retinal very sensitive to red light – When retinal separates from rhodopsin this action converts light energy into the electrical signal our brains interpret to see. While we don’t get much visible light at night, it turns out this mechanism can also be triggered with another combination of light and chemistry when we employ it.
Under infrared light and with a chlorin injection, retinal changes in the same way as it does under visible light.
This explains the increase in night-time visual acuity. As chlorin e6 absorbs the infrared radiation, it interacts with the oxygen in the eye tissue, transforming it into highly reactive singlet oxygen – as well as destroying cancer cells, by creating singlet oxygen. In cases where thiamine is deficient, more oxygen is available in the blood as shown above and an abnormally large ROS singlet signal is made and this can lead to more damage to photoreceptors. In cases, without significant thiamine deficits, this free radical can also react with retinal and enable a boost in night vision.
This is why in patients undergoing photodynamic therapy with cancer, many have reported seeing silhouettes and outlines in the dark. This example also illustrates how thiamine and retinal physiology are intertwined with light frequencies.

