Every wave connects with everything in nature via resonance and so it is with life. —- Dr. Jack Kruse
Is there something special about how the sun creates light that is critical for a mitochondriac in training to understand? Do you know what it is? If the blood plasma connected the sun with our mitochondria by way of hemoglobin, what is it about the sun’s light that makes it seem so cozy with our mitochondria? Could the sun be an object that uses magnetohydrodynamic equations to communicate directly with mitochondria and use our blood plasma as its conduit? It is a provocative idea for a conventional physicist and biologist. The interesting thing is, it explains many of the phenomena we see in life on this planet. The liquid plasma model of the Sun is a non-equilibrium approach to the problem of understanding how life came to be as it is. This idea supports the idea that the entire sun supports nuclear reactions and they are free to occur throughout the solar mass. The Standard gaseous model believes the sun is a layers of gas that looks much like the layers of an onion and uses “thermal emission mechanism” to explain how white light is created with a continual spectrum by the sun.

This standard model has never been able to explain why the surface temperature of the sun is what it is, and why light emission on Earth always is associated with some condensed matter lattice state. If the sun’s surface does acts like matter on Earth, and uses a lattice to emit light, we should expect that primary means of addressing internal heat transfer is based upon convection and conduction and not thermal emission. Is there evidence that contradicts the standard gaseous model of the sun? There is. How would these ideas mesh with biology? Is this new idea biologically plausible based upon what we know about the mitochondrial matrix? I believe it is. In fact, I believe bio-physics on Earth maybe the key for scientists to finally realize that creation of the sunlight might be the key in fully understanding the theory of evolution of all life on this planet. It may explain why Margulis, Woese, and Bill Martin’s key questions of describing why life is, the way it is.
So what about this theory is key for the mitochondriac to know? Today’s blog aims to discuss how this idea explains why the solar spectrum color palate is what it is, and why red light is the dominant form in solar light from sun up to sun down. Might we finally figure out why all the key chromophores tied to energy production inside a cell are all red light chromophores?
Let us begin.
Inside stars like the sun, the extreme temperature rips atoms into their components: protons, neutrons and electrons. Under normal conditions, the mutual repulsion of individual protons ought to force them apart. Quantum-tunneling effects in the sun allow hot, high-speed protons to fuse into helium nuclei. This fusion reaction drives the sun’s radiance and provides all the light and energy that fuels life on Earth.
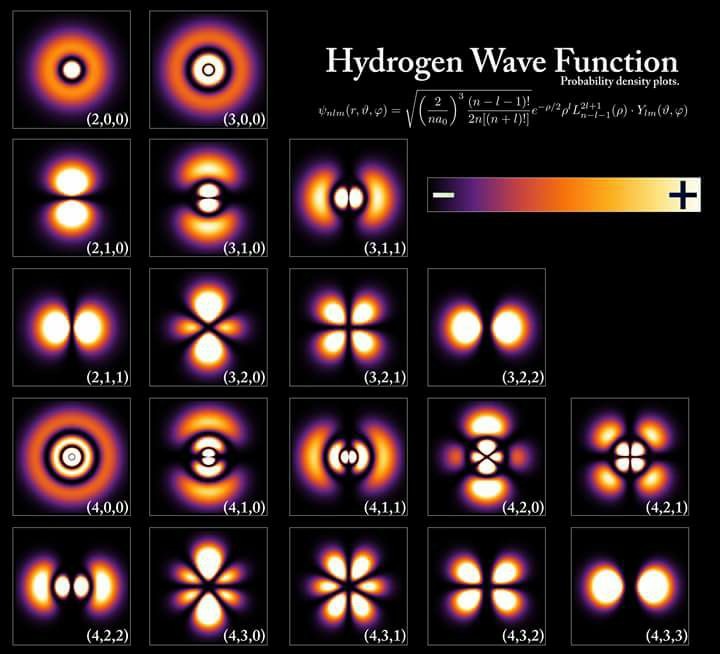
Hydrogen has many wave functions in our solar system as the picture above shows. So why is it that certain wave functions are favored by the sun? Is there some reason why one wave function leaves the rest in the dust?
The paradigm dominant in astronomy believes the sun uses a gaseous fusion model. The paradigm of how the sun operates is not critically important to accept or deny for the mitochondriac in training. The reason is simple. Life only relies on the light that comes to the Earth to drive evolution. So for the mitochondriac, the issue of who is right about the solar model is not material to the larger question, Why is life built as it is ? Right now, with my understanding of light emission, I don’t accept the Standard gaseous model of the sun. I want to share that bias with my readers. I think a lattice has to be present to create and absorb light. I believe this because that is the best evidence we have on Earth right now.
What is that evidence?
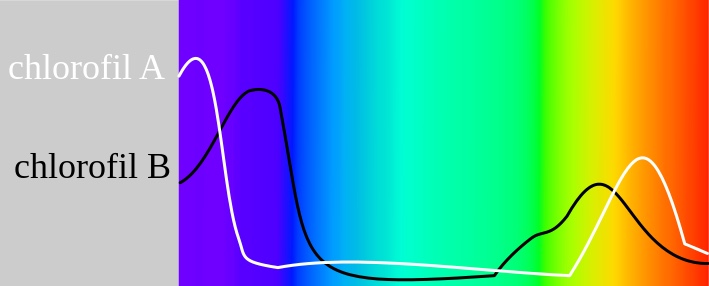
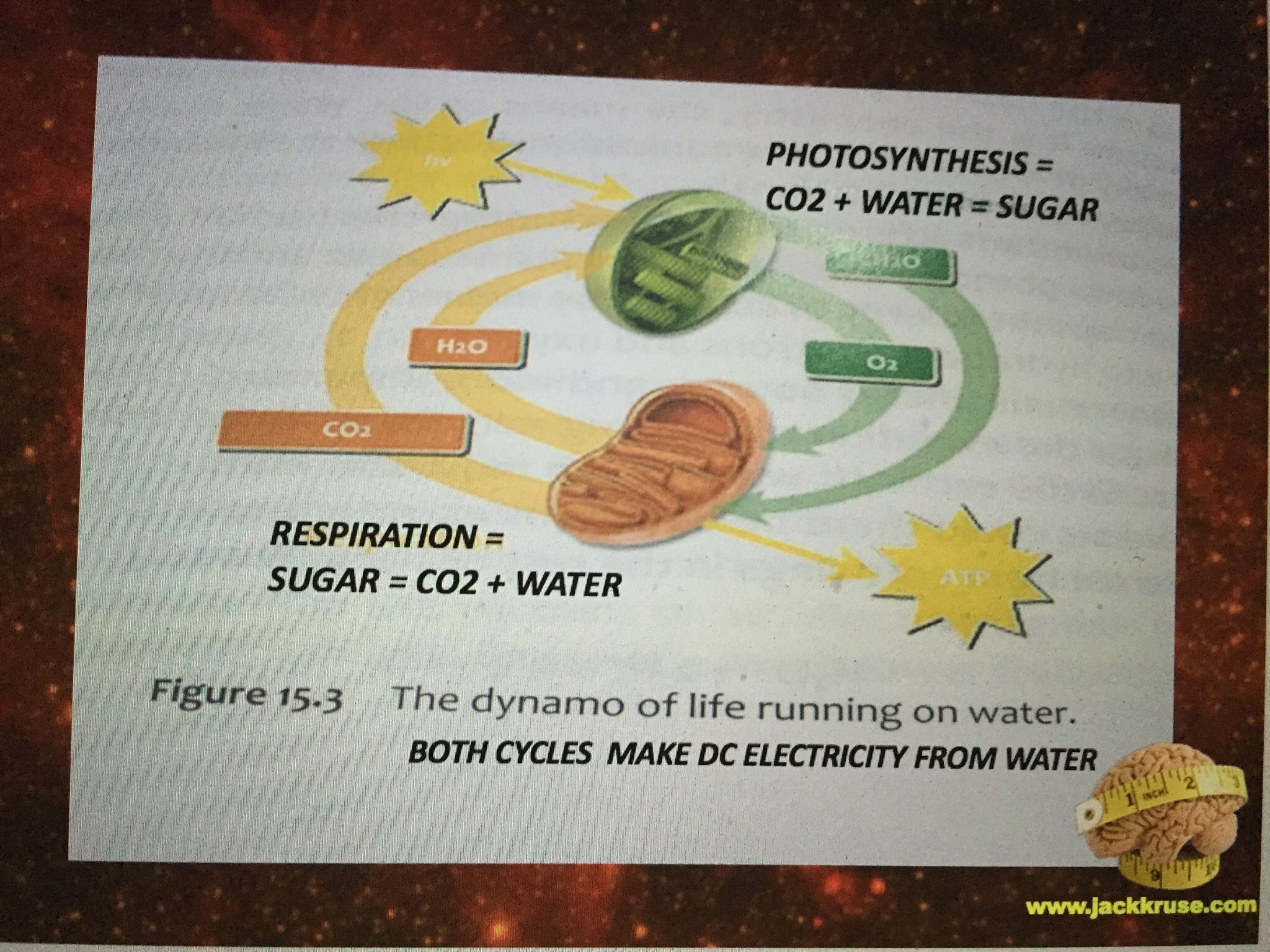
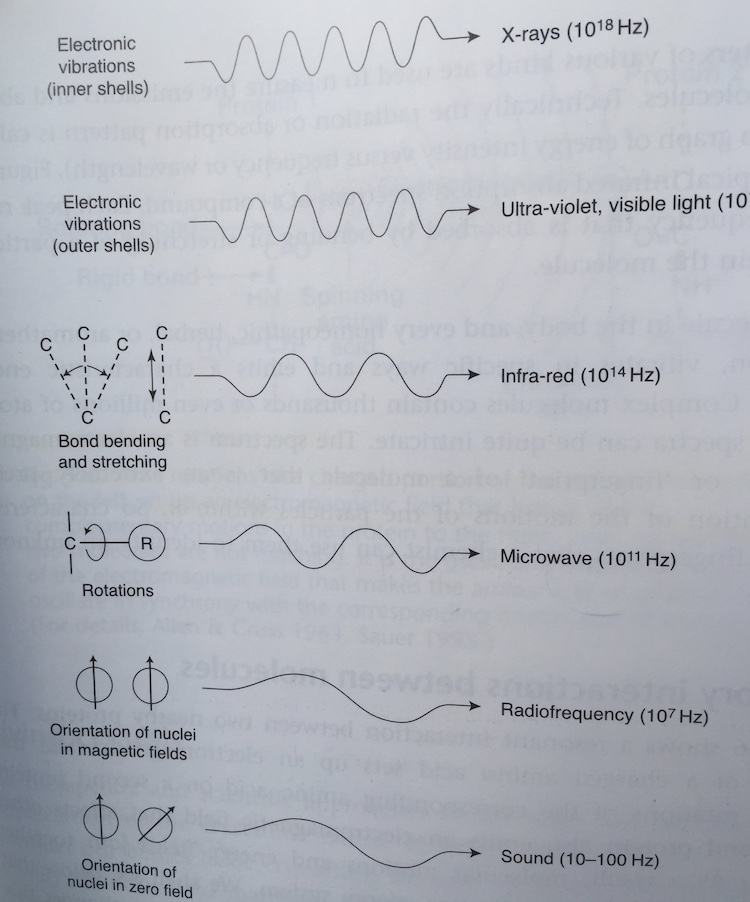
The evidence is in the evolutionary biology of the photosynthetic apparatus on Earth that uses sunlight to make energy for the enture food web and all domains of life. When evolutionary biologists began to look at the photo-chemical reactions, at first, most scientists did not believe that all the reaction centers found in photosynthetic organisms today could possibly have a single common ancestor. It is true, all reaction centers harvest energy from light and lock it into compounds in a form that’s chemically useful to cells. To do this, the proteins pass electrons along a transfer chain of molecules in a membrane, as though skipping along a series of stepping stones. Each step releases energy that’s ultimately used down the line to make energy-carrier molecules for the cell. In this way light excites electrons and then as the electron moves through out the living system it gives off its light to parts of the cell. This is best represented by the Jablonski diagram below.
In photosynthesis, in terms of function and structure, the photosystem reaction centers fall into two categories that differ in almost every way. Photosystem I serves mainly to produce the energy carrier NADPH, whereas photosystem II makes ATP and splits water molecules. Their reaction centers use different light-absorbing pigments and soak up different portions of the spectrum of the sun. Electrons flow through their reaction centers differently. And the protein sequences for the reaction centers don’t seem to bear any relation to each other at first glance. Remember photosynthesis showed up on Earth about 50 million years before mitochondria did.
Both types of photosystem come together in green plants, algae and cyanobacteria to perform a particularly complex form of photosynthesis. This is called oxygenic photosynthesis; it produces energy (in the form of ATP and carbohydrates) as well as oxygen, a byproduct toxic to many cells. The remaining photosynthetic organisms, all of which are bacteria, use only one type of reaction center or the other.
So it seemed as though there were two evolutionary trees to follow, that was, until the crystal lattice structures of these reaction centers began to emerge in the early 1990’s. This is when I got very interested in how the sun created its own spectrum of light. I realized immediately that photosynthesis was ancient compared to mitochondrial respiration. And if the photostem reactions used a lattice there might be a reason in the sun for doing so. I knew that light photons were the force carrier for the electromangetic force, so this use of molecular resonance to control proteins was present 4.4 billion years ago before ANY LIFE had evolved. In the 1990’s researchers began to see undeniable evidence that the reaction centers for photosystems I and II had a common origin. Again they were tied to a lattice structure, and all lattice structures are loaded with electrons. Photons interact with electrons via the photoelectric effect. Specific working components of the lattice centers seemed to have undergone various substitutions during evolution, but the overall structural motif at their cores was highly conserved. This told me the sun’s spectrum had to hold the key to this puzzle. It turned out that big structural features were retained in bio-molecules, but sequence similarities were lost in the mists of time. Then I realized why DNA and RNA only code for proteins. They were antenna’s for the solar spectrum.

What else is special about crystalline lattices for a mitochondriac to know?
The mitochondriac credo is: everything scales back to light, water, and magnetism……..
How does magnetism put its two cents into this solar and water story?
Did you know crystal are loaded with tons of electrons? Do you know where crystals naturally form in the universe because of the laws of electromagnetism? Crystals form along the lines of magnetic demarcation set forth by the magnetic field of an object. The Earth has its own magnetic field, and the sun also has massive magnetic fields. Moreover, so do chloroplast photo-stems, because of the presence of water (magnetic dipole) and spinning ATPase head creates a magnetic demarcation in a cell. These specific resonant frequencies in the crystals with these magnetic demarcation could draw light to these crystals by magnetic molecular resonance; This resonance would result in an electromagnetic collision creates a standing wave or an electromechanical deflection in the crystal to record information about the incident light wave. This wave forms along this magnetic demarcation lines, attenuating and collecting minerals of similar resonance created by the incident light EMF. Crystals are able to amplify those harmonics in solar light waves and tune them in their crystalline lattice to use them. Do you know what the harmonic of sunlight is? It is discussed later in this blog. You’re going to be in for a shock. In this way, you then see how the Earth magnetic field was critical in “tuning crystals formed in chloroplast membranes, (and RBC’s, and mitochondria) to create the photosynthetic cores on Earth. These photosynthetic crystalline cores makes the ENTIRE food web on Earth.
So do you still think food is foundational now?
Or is the 3 leged stool of the “mitochondriac” more educational?
Today, I think the sun creates and emits light using a lattice of condensed matter in the photosphere. From here, the sun’s light brings life the energy it requires to run our cells and all biochemistry. This is documented in the Jablonski diagram above. I believe it is biologic plausible to say the sun might use an electric model to explain the temperature of the surface of the sun, and the continous light spectrum it emits. I believe this because of what we have uncovered about the photosynthetic machinery of the chloroplast mentioned above. The interesting thing is, no matter the mechanism behind light creation in the sun, the actions of the atoms inside the sun is likely the same to create light. Take a close look at the picture at the beginning of the blog now. This lays the process out visually for you to see, and shows you all the abiotic atoms in the mix that help create light.
In the proton-proton fusion reaction, first two protons fuse. Usually the pair breaks apart again immediately, but once in a while one of the protons is transmuted into a neutron. The resulting proton-neutron pair is deuterium, a heavy type of hydrogen. Also, a positron and a neutrino are emitted. I spoke about neutrino’s in my April 2016 webinar on my website. When the positron encounters its antiparticle (an electron), the pair annihilates to form a gamma ray.
Then, another proton collides with the deuterium hydrogen nucleus, forming a helium-3 nucleus (two protons and a neutron) plus a gamma ray. Gamma rays eventually work their way up from the core of the sun and out into space in the form of a part of sunlight. Deuterium is a also known as heavy hydrogen. Deuterium is a key understanding in becoming a mitochondriac. It is the point of this blog.
The helium-3 nucleus collides with another one, creating a helium-4 nucleus, plus two extra protons.
Why do most humans get sleepy when the sun sets and awaken when it rises?
Have you ever been woken by someone turning on a light? Do you notice it’s harder to fall asleep when your television is on? If so, you have experienced a phenomenon present in all diurnal animals: Light helps to keep diurnal animals, those that are awake during the day and asleep at night, alert and awake throughout the daytime hours. This is true for humans, diurnal mammals and even many kinds of fish. But how exactly does light produce this effect on us?

Our bodies are set up to sense and respond to solar light in very unique ways because of the bio-molecules present in cells. Sunlight has a diurnal foot print in the morning that differs from dusk. That light fingerprint cause hydrated proteins in our eye, skin, and cells to vibrate in a specific way. In this way we can think of the sun as a giant drum that creates light that hits proteins to make a sound or quantum vibration when it arrives above the horizon. As the sun rises, the sun’s spectrum is continuously made, but its light varies diurnally on earth as the day evolves. This variation means that the standing waves from the sun vary in cells crystals as the sun waves vary. This diurnal variation is regular because of the physics of Earth make for a specific periodicity. This specifificity is what sets all circadian signaling in our bodies. All proteins that work in these cycles with sunlight are linked by the frequency of light that is dominant at that specific part of the day. For example, when blue light rays are declining quickly in the 435-465nm range, evolution built an opsin called melanospin to be a detector for this specific frequency declining. When light is sensed by cells in our retinas by melanopsin, signals are passed to the suprachiasmatic nucleus (SCN), a part of the hypothalamus of the brain that integrates signals and serves as the master clock of the circadian rhythm.
Now in a previous blog I said, “Modern physics cannot figure out why the corona is as hot as it is, based upon Kirchoff’s law. Why should you care? Anything that affects how the sun makes light, effects our understanding of how circadian mechanisms and epigenetic mechanisms works in living systems. That is why this topic is not esoteric to a mitochondriac.”
Why did I say it?
If you look at the picture at the beginning of the blog you’ll notice something unusual about the atoms in the sun. When hydrogen protons in the sun fuse they make deuterium.
Hydrogen is the simplest atom, which consists of one proton and one electron while deuterium is made up of one neutron, one proton, and one electron. Since the physical properties indicate that hydrogen and deuterium are very similar, one would expect their wavelengths to be very similar. Research has shown, the calculated data of the three of the visible wavelengths in the hydrogen spectrum to be 656.478 nm, 486.542 nm, and 434.415 nm. For deuterium the calculated wavelengths shift to 656.296 nm, 486.409 nm, and 434.295 nm respectively due to the additional mass in the neutron in the nucleus.
A normal atom, with equal numbers of protons and electrons, is electrically neutral. You get no electric shock by touching matter in its normal state. It is easy, however, to remove electrons from an atom and to electrically unbalance it, especially in the sun’s immense electrical and magnetic fields. The result of electron removal is a positively charged “ion.” In fact, all the electrons can be removed to reveal a bare nucleus. Hydrogen, with one electron and one proton, has but one ionization “state” = the lone proton we call H+
Deuterium is an isotope of hydrogen. This means it has a different atomic mass than the lighter version of hydrogen (H+). Deuterons also have a different quantum spin number from H+. This is big deal too. The deuteron of hydrogen is made of a proton and a neutron slammed together in the solar mass. This means deuterium is double the atomic weight of H+, or lighter hydrogen. The charge of each of the atom’s isotopes are the same, but their masses vary, and are significantly different as are their frequencies of light they emit. It has been found the spectral emission of deuterium to be 1.65%, 5.3%, and 2.5% for the wavelengths of red, blue, and violet respectively.
Now, to get to the heart of the matter, how does free-flying radiation interact with atoms to give us detailed information about stars and other celestial bodies? The light send radiation fingerprints from a hot, incandescent solid through a gas of low density and we observe what happens in a lab. The electrons that surround an atom have a minimum energy below which they cannot go (a discovery of “quantum mechanics” in 1930’s). The electrons will naturally seek this lowest energy level. If you move the electrons outward, away from the nucleus, you give them more energy. However, electrons are very specific about what energies they will take on. There are quantum rules for this in nature. For any given atom or ion, only certain specific electron energies, that is, specific energy levels, are allowed. We call this a quantized function. Electrons can be moved from one energy level to another by collisions among atoms or by absorption of photons. However, an electron in a specific level cannot absorb part of a photon, but must absorb all or none of it. As a result, only photons with particular energies, those that correspond to differences between the various energy levels, can be absorbed from the flow of passing radiation. This is the basis of how light can control atoms or molecules using their electrons. We call this molecular resonance. Since photon energy corresponds to wavelength, only specific wavelengths (or colors) can be absorbed. And since the electron structures are different for each kind of atom or ion, the photon energies that each kind will absorb are also different. When we look at the spectrum from the hot source after it has gone through the low-density gas, we therefore see narrow gaps at particular wavelengths where the light is diminished or even gone altogether. Because of the way they appear, these gaps are called “absorption lines.” Each atom or ion has a unique set of absorption lines in our sun. Hydrogen has only four in the visual spectrum: at wavelengths of 6563 A in the red (called H-alpha), at 4861 A in the blue (H-beta), and at 4340 A (H-gamma) and 4101 A (H- delta) in the violet). See below

Why am I asking you to pay deep attention to this?
Deuterium is destroyed in the interiors of stars faster than it is produced!!
In our sun (G class star), on the time scales of its creation, deuterium hardly exists at all. In fact, in the corona/photoshpere of the sun, deuterium is almost non existent. This occurs because of how the atoms collide by the solar fusion mechanism. This means because deuterium is short lived in our sun, and it means it rarely gives off its light emission signature in the sun’s light. This means its corresponding light would rarely reach Earth’s surface. If it can’t reach Earth how could it control deuterium on Earth? Remember light can control atoms or molecules it resonates with. This is a nuclear magnetic effect.

Where is most deuterium on Earth found? Look up. It is found in sea water in the oceans. This is also where life began.
So, if deuterium’s light emissions rarely could get to Earth, could atoms on the surface of Earth have used deuteriums’ light as the basis of redox chemistry with the energy from sunlight from our star?
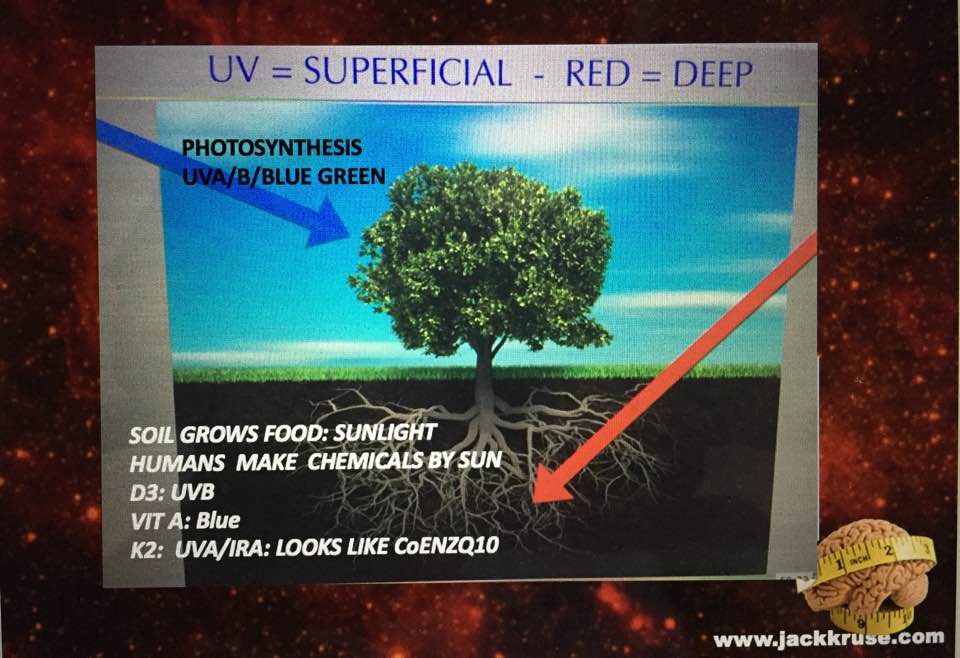
The answer is NO. By nature’s design, stars destroy deuterium nucleons faster than they are made. What are the two things on Earth that living systems use to colllect light to drive reactions of biochemical pathways? Chloroplasts and mitochondria. One consumes water, and the other MAKES water. Water is two part hydrogen and one part oxygen. Now for a biology lesson.
What is the most ancient parts of cells in all 3 domains of life, found in both chloroplasts and mitochondria? ANSWER: The ATPase was built before life was. All the ATPase needed to function is an associated lipid membrane to separate protons and a source of protons under the power of red light to spin its rotary motor. Then energy could be made form the sun on Earth. It has already been established by science that the ATPase works at 100% efficiency in red light. Look below.

Look at the first line of the slide above: All evolutionary biologists know today, this statement is factual. In fact, in 1978 we gave Mitchell a Noble Prize, mainly because Mitchell’s science showed that proton gradients are ubiquitous in all three domains of life. They are more ubiquitous than even DNA and RNA. That is a huge statment. Proton gradients are critical in energy production in all living things on this planet. No question about it, protons are that big a deal to living systems. Did Mitchell have it all right and perfectly worked out? No. Mitochondriacs know about the work of Dr. Gilbert Ling and why he had a big problem with Mitchell. Mitchell’s ideas never incorporated how sunlight’s energy might also be stored in water’s lattice (EZ) to help augment the proton gradients. This idea would help to explain the enzymatic flux in cells to satisfy the laws of thermodynamics. This is why Ling was furious with science for 60 years.

With this idea, Mitchell’s work broke the second law of thermodynamics by 500% (Ling et al) Mitchell ideas only focused on just one energy source, the proton gradients. Ling pointed out that focusing in on one part did not explain the energy needed to run life in the 1960’s and was ignored. His calculation of enzymatic flux showed something else had to be at play to explain the energy deficit. We found out in 2000 (Preparta/Del Guidice), and in 2013 (Pollack et al.) that sunlight can make a battery in water via charge separation and create a redox pile of electrons in coherent domains of water’s lattice. This mimics what scientist found out in the 1990’s about the photosyntheitc mechanism as well. i will remind you now that the first step in photosynthesis is to charge separate water into H302 and H+. H+ is the FOCUS of this blog. What does this mean? Sunlight burns water to make H+. The H+ the sun makes depends upon the concentration of of hydrogen isotopes in the water the sun hits. Sea water has 155 parts per million of deuterons inside of it today. Was this always true on Earth? I do not believe it was.
Let us get to the proton gradients of Mitchell.
Now, 4.4 billion years ago when the sun began to shine, what was on the surface or mantle of Earth at that time? What do we definitely know about Earth back then? What were the thermodynamic givens? We know the Earth had water then. Was the water then the same as it is today? We also know from sediments the Earth had a lot of CO2.

Sunlight releases photons. Sunlight can shine in the bottom of an ocean because the heat from the oceans vents is still behind the power source of this heat. Heat is a form of light. Photons are the force carrier for the electromagnetic force. Light controls charged particles. This means that light is the ONLY thing that can control charged sub atomic particles in the universe. What kind of light does our ‘G class star’ emit mostly again? RED LIGHT. Where does it come from? H+. When Hydrogen is ionized it loses it electron and become H+ and it glows red.
H+ = a single light hydrogen proton. It is also positively charged. This means that red light photons from the sun can control and program things with H+ in it. This is how nuclear and molecular resonance with light works. Light is capable of controlling all things with charges including the larger bio-molecules found on a planet by using light frequency in the emission spectra of H+ from a near by star to energize electrons and protons in many ways. I showed you in Reality 16 all the different ways it was possible, but here in Reality 18, I am telling you how RED light controls H+ in all things, because ionized H+ is where all the red light is emitted from in the photosphere of the sun.
What are the implications for atoms on Earth then? Did this single step inside a star dictate why the first complex life molecule had to work exclusively with H+ and not deuterium? The molecular signals emitted from atoms or chemicals acquires its electromagnetic meaning only when light interacts with it in some way. As I’ve mentioned several times now, light effects things with charges and with mass. Electrons are negatively charged and are effected by the photoelectric effect. What about protons? They are positively charged. What about deuterons? They have the same charge as H+, but they have a massive increase in mass and spin. This means they also have a different nuclear magnetic moment. What does red light from the sun do to protons? It can control them because of these differences in the hydrogen isotopes.
What element is dominant in the photosphere? Light hydrogen is. We call this H+, or protium. Please go right to 3:50 of the video below and watch it carefully before continuing.
The above video shows you where 42% of the sun’s red light comes from. It comes from H+ nucleons that get ionized in the photosphere of the sun’s corona to make the 42% of red light. This means several key things to living things with a chloroplast of a mitochondria on Earth. One, it means that H+ in the corona is the source of our red light spectra from the sun on Earth. 2. Red light controls the behavior of H+ on Earth. This means that the red light emitted from the sun can be used to control things with H+ in them at any distance away from the sun. The mechanism that controls this reaction is molecular resoance which you learned about earlier in the series, called molecular resonance.
What two key things contain H+ in living systems?
Chloroplasts and mitochondria.
Anything else?
WATER.
I’ve told my mitochondriacs in training everything about life comes back to light water and magnetism. And now I am proving it to you.
When sunlight hits water what does it exclude? Protons. It separates H+ and deuterons from liquid sea water to make an exclusion zone doesn’t it?
Is the EZ of deuterons the same as the EZ from H+? It is not. Sadly Pollack has not re done his experiments using D20 over H20. D20 viscosity is much higher than H20. This increase in viscosity would make the ancient ATPase spin less fast to make less energy. Before life existed the thermodynamics of energy would not have been favorable to use deuterons for this reason.
Red light is capable of moving things with mass. Since deuterons are heavier than H+, red light would be able to move them less, thereby delivering less energy than H+. These two thing created a great thermodynamic reason to use H+ over deuterium in the seas, even thought deuterium was very common in the ancient seas. The primordial water on Earth is believed to come from comets and asteroids which has water with high deuterium content.
At this point I want to remind you about a comment I have made many times over the last few years in blogs, social media, and the forum. I have told you that the sun and mitochondria are quite similar in many ways that are not always obvious. The mechanism I just mentioned is WHAT I HAD in mind when I said it. Our sun makes red light because the lattice in the corona FAVORS the creation of H+ over deuterium. This means the sun’s continuous spectrum has a favorite isotope of hydrogen, and more importantly it can control it. If it can harness it would it have chosen it naturally to build its own nano-ratotry machine to make energy from sunlight?
It turns out, it is the sole proton of hydrogen, stripped of its only electron, is what our star can control very well in wireless fashion using the red light of our star. This is the very same form of hydrogen we see in the mitochondrial matrix of eukaryotes and in chloroplasts. Chloroplast consume water and sunlight while mitochondria make water and CO2. (Slide Ve
rmont 2017)
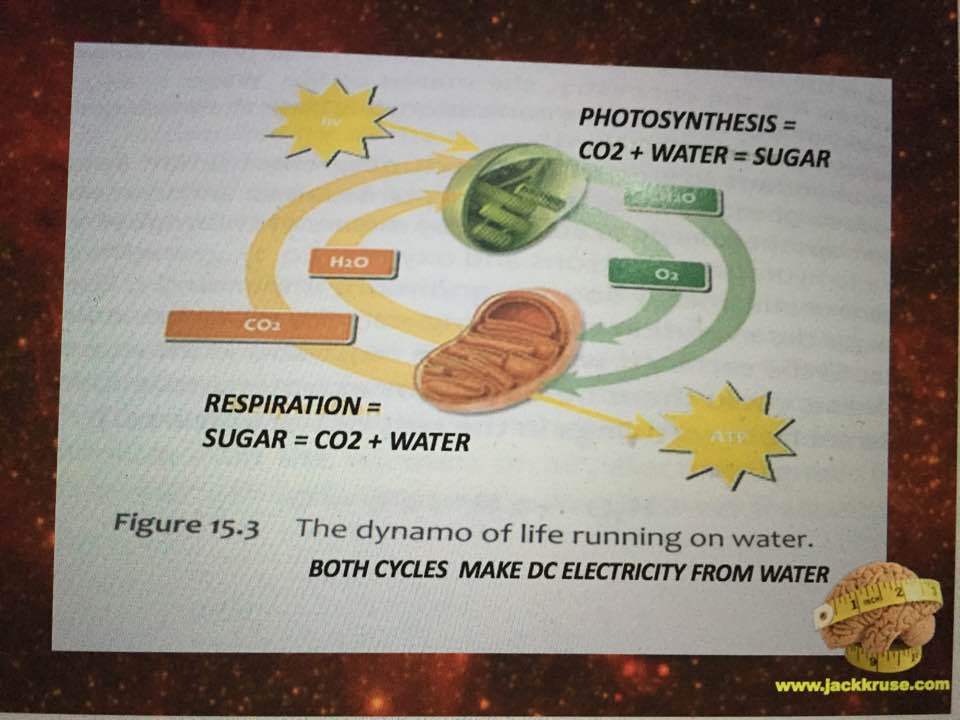
What does this all imply to a mitochondriac?
It tells you this is where natural selection and conditions of existence really began on Earth. Did you know one of the first bio-molecules built on earth before life was the ATPase? Check out Bill Martin’s work if you doubt me. That ATPase is the 5th cytochrome of all mitochondria on this planet, FYI. Did you know that this bio-molecule motor works best with H+ and red light? Its efficiency has been measured at 100%. Fact check that. In fact, did you know that the ATPase and all the water that surrounds it in a cell are also red light chromophores? Pollack showed us water absorbs from 270 nm all the way to 3300nm. And it absorbs best in the 600- 3100 nm range. Did you know 1535.8 nm light control protons best? Mae Wan Ho published this. Did you also know that the cytochrome adjacent and located just before the ATPase, called cytochrome c oxidase, is also a red light chromophore that also uses H+ exclusively? It also has a specific UV and IR spectrum.
Today, we know 100% for sure that H+ is a key part of the photosphere of the sun. We don’t yet realize a condensed lattice is likely where the red light is emitted but that is not the key for biology. The production and presence of red light is. This is why red light is now considered the best drug for humans by some of us. Moreover, it appears the production of red light by our G class star might be the reason life selected “light hydrogen” use over deuterium on Earth. My bet is that all the planets in the heliosphere of the sun would have to make the same choice because water and methane is where most planetary hydrogen is locked up.
Why? Take a look at the picture at the top of the page again.
The main fusion reaction in the sun is the proton-proton chain reaction, which takes six protons and produces two protons, one alpha particle, two anti-electrons, and two electron neutrinos. Here comes the key point of this entire blog entry: .
The deuterium nucleus is only barely bound and can be destroyed immediately— dissociated into a proton and neutron — by absorbing a gamma ray with energy more than 2 MeV. This means that all of the sun’s “primordial” deuterium, formed in the first minutes after the Big Bang, was destroyed before the sun got hot enough to begin fusing atoms on its own. What this means is that essentially all of the deuterium in the sun exists ONLY as an intermediate step in the proton-proton fusion cycle. This is why life is built the way it is.
This was the question set forth in Nick Lane’s book, The Vital Question.
To be complete in this story: tritium is another form of hydrogen made in the sun but it is naturally unstable, with a half-life of only 12 years. This means that any tritium in the sun (or anywhere else in the cosmos) has been mostly produced in the past few dozen years. On Earth, tritium is produced by cosmic rays interacting with stable matter. We see this in auroras in the ionosphere or in lightening storms. In the sun, most of the tritium would come from neutron capture on deuterium nucleons. This is where I suppose the neutrons would have come from dissociated deuterium or nucleon-exchange or proton-knockout reactions on helium-3 and helium-4.
What is my proof in this conjecture? What then are the mass fractions that make up the sun?
H+ is the dominate atom in our star as can be seen above. When H+ is ionized by plasma it emits red light. What does the solar spectra look like when it is broken down by a prism?
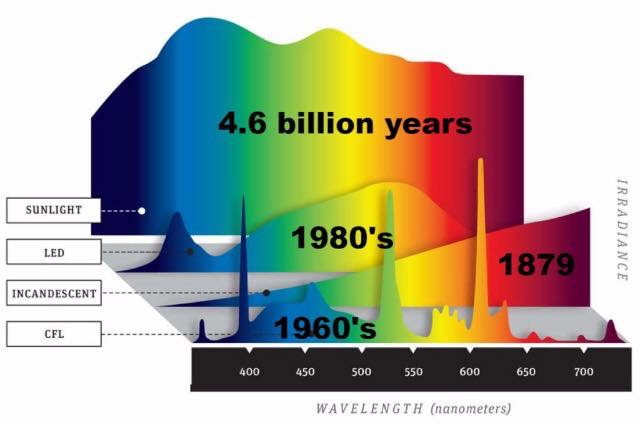
Note the dominant part of the solar spectra is RED light made by the H+ ion.
This blog tells you why the first step in controlling the thermodynamics on Earth was to use the most common element in the sun’s spectra. It’s emitted photons acted as the controling arm of H+ on Earth. When you look inside a chloroplast of mitochondria the evidence is everywhere when you observe what nature is telling us.
It also explains why we got natural selection and conditions of existence as the first steps in evolutionary change. The problem is, it was not Darwin’s version……..it is a quantum selection made by H+ light emission.
But what else does it mean?
The sun is the center of the quantum vibrations in our solar system and at 93 million miles from the sun, the Earth sits at the 3rd harmonic of the solar plasma in our planetary system. The frequency of solar plasma at our distance is around 3 mHz (milli Hz).
Why did I mention that about the sun?
There is lots of interesting things to say about blood cells and fluid flow in humans. There is a new field of electro hydrodynamics, where they study the geometrical structure of blood cells in our circulation, and electron flow might be the key to how the sun interacts with them. I quote, “the 3rd lamellar spacing of RBC’s = 40.6 Å (the 3rd, core hydrophobic lipid layer is significantly smaller than the spacings above, and the electron density is almost constant in the hydrophobic membrane core. This density profile is well described by α-helical coiled-coil peptides, which are embedded in the cell membranes.” They go on, “hexagonal packing of the lipid tails are in the hydrophobic membrane core. The distance between two acyl tails has been determined, where q|| is the position of the corresponding correlation peak. We note that this area also includes the area of cholesterol molecules. Where did this come from? Right here: http://www.nature.com/articles/srep39661
What did that just mean for the mitochondriac in training? It means I just showed you how we are supposed to connect with the sun. The picture below illustrates the complex science for you.

STAY TUNED for more of how light of our star controlled the story of how protons control life on Earth. I’ll end with this quote because who said it will continue the proton story and be critical for the November 2017 webinar for members:
Food and light are energy.
“We live by a small trickle of electricity from the sun.” The green of our garden, the algae in our water, the trees, grasses and herbs on our lands are the transforming agents that harvest the sun’s light via the process of photosynthesis. When we consume these foods, this stored sun energy is released into our bodies as electrons and protons; it is then transformed into ATP, adenosine triphosphate, the biological energy necessary for all cellular function.”
– Szent Gyorgyi













 Everyone thinks social media is great……….what if they are wrong?
Everyone thinks social media is great……….what if they are wrong?